Advances in Preclinical fMRI
Xin Yu1
1Massachusetts General Hospital and Harvard Medical School, United States
1Massachusetts General Hospital and Harvard Medical School, United States
Synopsis
Keywords: : Preclinical/Animal, Image acquisition: Fast imaging, Contrast mechanisms: Non-proton
In this course, I will discuss the ultra-high resolution preclinical fMRI methods: line-scanning fMRI and single-vessel fMRI. In particular, the k-space reshuffled FLASH fMRI method will be highlighted to specify its unique features for high spatial resolution (vessel-specific) mapping and high temporal resolution (~5-10ms TR) mapping. Also, awake mouse fMRI with 100umx100umx200um resolution using 14T MRI scanner will be presented, which can be further applied to specify brain-wide vasodynamic changes. In the end, I will introduce the feasibility testing of 23Na-fMRI in rodent brains."Functional"
MRI is developed to map neurovascular coupling-based hemodynamic changes, i.e.
the cerebral blood volume (CBV), cerebral blood flow (CBF), and blood-oxygen-level-dependent
(BOLD) signals, as indirect measurements of neuronal activity. Despite existing
functional mapping studies on the spatial specificity of fMRI signal to its
neuronal sources, one intriguing question is “what can we detect when the
spatial resolution is improved from the millimeter to the tens-of-micron
scale?”
Here, I will present two sets of high-resolution fMRI methods: line-scanning fMRI1 and single-vessel fMRI2, using the 14T MRI scanner. The line-scanning fMRI allows laminar hemodynamic mapping with 50-micron resolution and 5-to-50ms sampling rates. Given the 2mm thickness of the rat cortex, the laminar-specific fMRI signal can be sampled across over 40 voxels according to different cortical layers, as well as the white matter tract, i.e. corpus callosum. The ultra-fast sampling rate also helps eliminate any temporal aliasing effect due to the heartbeat or other non-physiological oscillatory noises. The line-scanning fMRI includes two sets of mapping schemes: gradient echo-based line scanning with saturation slices, and spin echo-based beam projection. This method presents advantageous laminar fMRI mapping capability in human brains. Single-vessel fMRI was first presented by alternating k-t space reconstruction of 2D FLASH time series to achieve ultra-high resolution T2*-weighted images with 50-100ms temporal resolution. The single-vessel fMRI enables the detection of arteriole and venule (20-70 micron)-specific hemodynamic responses across different brain regions in animals. Also, balanced steady-state free precession (bSSFP)-based single-vessel fMRI was developed to map the resting state BOLD and CBV signal fluctuation from individual penetrating vessels, enabling the investigation of the vasodynamic changes, e.g. vasomotion, non-invasively. This method can be readily applied to detect altered neurovascular coupling events in combination with fiber photometry-based Ca2+ or Glu recordings in deep brain regions of animal models with brain injury or neurological disorders. We also applied 14T to detect the phase-contrast-based functional cerebral blood flow (velocity) changes from individual penetrating vessels3, providing a quantitative measurement of blood velocity in micro-vessels with fMRI directly.
Our latest effort includes the development of high-resolution Echo-planar-imaging (EPI) to achieve the 100um isotropic resolution fMRI images from awake mice. This method enables the brain-wide vascular dynamic mapping in both task and resting states of behaving animals, shedding the light on dissecting the vasodynamic changes coupled to neuromodulation in deep brain functional nuclei. It should be noted that to achieve high signal-to-noise (SNR) ratio, we implanted a surface transceiver coil that is directly attached to the animal skull. This surgical procedure enables the boost of SNR signal from the cortex, enabling the dynamic mapping of the x-nuclei signals, e.g. 23Na, for functional mapping, as one of the ongoing efforts in Yu lab.
Here, I will present two sets of high-resolution fMRI methods: line-scanning fMRI1 and single-vessel fMRI2, using the 14T MRI scanner. The line-scanning fMRI allows laminar hemodynamic mapping with 50-micron resolution and 5-to-50ms sampling rates. Given the 2mm thickness of the rat cortex, the laminar-specific fMRI signal can be sampled across over 40 voxels according to different cortical layers, as well as the white matter tract, i.e. corpus callosum. The ultra-fast sampling rate also helps eliminate any temporal aliasing effect due to the heartbeat or other non-physiological oscillatory noises. The line-scanning fMRI includes two sets of mapping schemes: gradient echo-based line scanning with saturation slices, and spin echo-based beam projection. This method presents advantageous laminar fMRI mapping capability in human brains. Single-vessel fMRI was first presented by alternating k-t space reconstruction of 2D FLASH time series to achieve ultra-high resolution T2*-weighted images with 50-100ms temporal resolution. The single-vessel fMRI enables the detection of arteriole and venule (20-70 micron)-specific hemodynamic responses across different brain regions in animals. Also, balanced steady-state free precession (bSSFP)-based single-vessel fMRI was developed to map the resting state BOLD and CBV signal fluctuation from individual penetrating vessels, enabling the investigation of the vasodynamic changes, e.g. vasomotion, non-invasively. This method can be readily applied to detect altered neurovascular coupling events in combination with fiber photometry-based Ca2+ or Glu recordings in deep brain regions of animal models with brain injury or neurological disorders. We also applied 14T to detect the phase-contrast-based functional cerebral blood flow (velocity) changes from individual penetrating vessels3, providing a quantitative measurement of blood velocity in micro-vessels with fMRI directly.
Our latest effort includes the development of high-resolution Echo-planar-imaging (EPI) to achieve the 100um isotropic resolution fMRI images from awake mice. This method enables the brain-wide vascular dynamic mapping in both task and resting states of behaving animals, shedding the light on dissecting the vasodynamic changes coupled to neuromodulation in deep brain functional nuclei. It should be noted that to achieve high signal-to-noise (SNR) ratio, we implanted a surface transceiver coil that is directly attached to the animal skull. This surgical procedure enables the boost of SNR signal from the cortex, enabling the dynamic mapping of the x-nuclei signals, e.g. 23Na, for functional mapping, as one of the ongoing efforts in Yu lab.
Acknowledgements
This research was funded by NIH funding (RF1NS113278, R01NS124778, R01NS122904, R01NS120594, R21NS121642), NSF grant 2123971, and the S10 instrument grant (S10 MH124733–01) to Martino’s Center.References
1 Yu, X., et al. . Nature methods 11, 55-58, (2014).
2 Yu, X. et al. Nature methods 13, 337-340, (2016).
3 Chen, X. et al. PLoS Biol 19, e3000923, (2021).
Figures
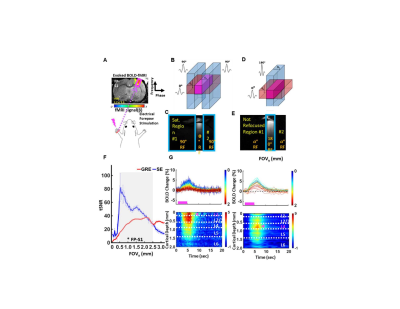
Figure
1. Gradient-echo and
Spin-echo based line-scanning fMRI (A-F). The line profile is detected by
turning off the phase-encoding gradient. The averaged tSNR across the cortical
layers is presented in F for both GE and SE line-scanning methods. The evoked BOLD signals are presented across
different cortical depths in G. (Yu et al. Nature Methods, 2014, Choi et
al. JCBFM, 2023).
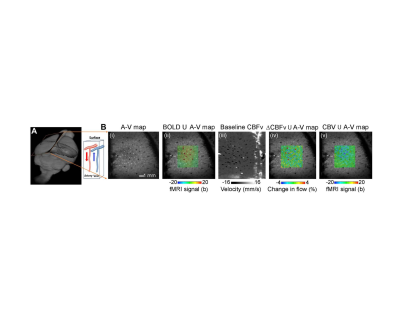
Figure
2. Single-vessel BOLD, CBV, and CBFv fMRI. A. The 2d-slice alignment to
extract arteriole and venule map (A-V map). B. The single-vessel BOLD (venules)
and CBV (arterioles) functional map with PC-based CBF functional maps. The
blood velocity map of arterioles and venules has opposite signs of signal
changes (adapted from Yu et al., Nature Methods, 2016, Chen et al. Plos Biology, 2021)