Imaging Markers of Neuroinflammation
1Department of Innovative Biomedical Visualization (iBMV), Nagoya University, Nagoya, Japan
Synopsis
Keywords: Neuro: Nervous system
Neuroinflammation, an immune response in the central nervous system, affects neuronal function. By using neuroimaging techniques such as SPECT, PET, and MRI, markers of neuroinflammation such as microglia activation, blood-brain barrier disruption, and interstitial fluid dynamics can be detected and monitored. These imaging markers are useful in diagnosing and tracking the progression of diseases such as multiple sclerosis, Alzheimer's, and Parkinson's disease.Take-home messages
1. Neuroinflammation is an immune response in the CNS, impacting neuronal function and often associated with neurodegenerative diseases.2. Neuroimaging techniques using SPECT, PET and MRI can detect and monitor neuroinflammation process through markers including microglia activation, BBB disruption or interstitial fluid dynamics.
3. Imaging markers of neuroinflammation have applications in diagnosing and monitoring diseases like multiple sclerosis, Alzheimer's, and Parkinson's disease.
Abstract
Neuroinflammation is a complex process involving activation of the immune system in the central nervous system. The term of neuroinflammation was initially used to refer to the accumulation and activation of microglia or the release of inflammatory factors seen in condition such as multiple sclerosis and neuroinfectious diseases. Recently, the term neuroinflammation has also been used to refer to the abnormal activation and response of microglia, which leads to excessive release of factors causing neuronal damage and loss of neuroprotective function, as well as other phenomena that result in an impaired perineural environment. Inflammatory processes takes places in most neurological disorders including traumatic brain injury, stroke, multiple sclerosis, Alzheimer's disease, Parkinson's disease.The development of biomarkers for Neuroinflammation was preceded by PET and SPECT compared to MRI. The translocator protein (TSPO) is a mitochondrial protein associated with neurodegeneration and has been linked to redox homeostasis. TSPO is upregulated during neuroinflammation, making it a biomarker. PET or SPECT imaging using TSPO ligands has revealed increased TSPO expression in animal models of neuroinflammatory conditions. TSPO PET signal was reduced in preclinical trials of novel therapies for HD and AD, suggesting its potential as a tool to monitor treatment response in clinical trials (1, 2).
There have been several attempts to develop methods for monitoring neuroinflammation using MRI. There are several attempts to monitor the neuroinflammation. The evaluation of blood brain barrier (BBB) integrity is one of the methods for the assessment of the neuroinflammation using MRI since most neuroinflammatory stimuli affect BBB integrity. Contrast-enhanced MRI (CE-MRI) is the most commonly used non-invasive imaging method to assess BBB alterations, and one of typical application of CE-MRI is the monitoring of inflammatory activity in multiple sclerosis (MS). Dynamic contrast enhancement (DCE) MRI is a common imaging technique used to measure BBB permeability by calculating a transfer coefficient (Ktrans). Alzheimer's disease (AD) leads to a gradual breakdown of the BBB, allowing toxins, inflammation, and pathogens to enter the CNS. Patients with AD show increased Ktrans compared to healthy aging. Recent studies show that changes in BBB occur before the onset of AD(3).
A study used ultrasmall superparamagnetic particles of iron oxide (USPIO) MRI to track phagocytic activity in mice with cerebral ischemia. An inflammatory response in the ischemic lesion and contralateral hemisphere was shown with USPIO-related signal spreading from ipsi- to contralateral hemisphere. Histochemical analysis confirmed inflammation remote from the lesion and nanoparticle ingestion by microglia/macrophages (4). MRS studies of CNS inflammatory and infectious diseases indicate elevated levels of myo-inositol (mI) and choline-containing compounds due to neuroinflammation. mI is a selective glial marker present in astrocytes, and its increase has been detected in both active and non-active phases of multiple sclerosis (MS) (5).
Neuroinflammation affects the function of the glymphatic system, a pathway that clears waste and solutes from the brain. Inflammation can alter the status of interstitial fluid dynamics, reducing its efficiency in clearing waste and increasing the risk of neurodegenerative diseases. One of the MRI method for evaluating glymphatic function is the intrathecal injection of GBCA and monitoring its clearance from the brain (6). Another approach for interstitial fluid dynamics is the diffusion tensor imaging analysis along the perivascular space (DTI-ALPS) method which is a non-invasive MRI technique that allows for the assessment of glymphatic function by measuring water diffusion along the perivascular space (7).
Acknowledgements
No acknowledgement found.References
1. Werry EL, et al. Int J Mol Sci. 2019;20(13).
2. Tournier BB et al. Cells. 2020;9(9).
3. Lee RL et al. Front Aging Neurosci. 2023;15:1144036.
4. Wiart M et al. Stroke. 2007;38(1):131-7.
5. Kirov, II et al. J Neurol Neurosurg Psychiatry. 2009;80(12):1330-6.
6. Ringstad G et al. JCI Insight. 2018;3(13).
7. Taoka T, et al. Jpn J Radiol. 2017;35(4):172-8.
Figures
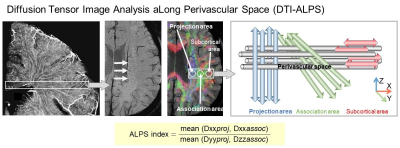
The DTI-ALPS method, a potential biomarker of Neuroinflammation
The diffusion tensor imaging analysis along the perivascular space (DTI-ALPS) method provides the ALPS-index, which is the ratio of the water diffusivity in the direction of perivascular space. It has been shown to reflect interstitial fluid dynamics in reports of Parkinson's disease and other disorders, and may be one potential biomarker reflecting abnormal interstitial fluid dynamics associated with neuroinflammation.