Measuring the Metabolomic Signature of Cancer
Marie-France Penet1
1Johns Hopkins School of Medicine, United States
1Johns Hopkins School of Medicine, United States
Synopsis
Keywords: Cross-organ: Cancer, Contrast mechanisms: Spectroscopy
Ex vivo MRS of cancer cells, xenografts, human cancer tissue, and biofluids is a rapidly expanding field that is providing unique insights into cancer metabolism. The field has been evolving as a stand-alone technology, as well as a complement to in vivo MRS to characterize not only the metabolome of cancer cells, but also of cancer-associated stromal cells, immune cells, tumors, and biofluids. The presentation will provide an overview of the insights into cancer obtained with ex vivo MRS and of future directions. High resolution MRS studies of cells, tumors and biofluids will be discussed, in preclinical and clinical settings.With its minimal sample preparation requirements, reproducibility, and quantitative ability, ex vivo MRS is a technique of choice for investigating the metabolome of cells, tissues, and biofluids in cancer. In an MR spectrum, each peak has a characteristic chemical shift defined in parts per million that is dependent upon the compound chemical structure, and a peak area proportional to the number of nuclei that can be used to derive metabolite concentration. Most studies are performed with 1H MRS because of its higher sensitivity compared to other nuclei such as 13C or 31P. During the presentation, different MRS applications will be described, including studies of genetically engineered cell lines with specific pathways silenced or overexpressed to highlight the impact of specific pathways on the metabolome. Results obtained with different cell lines or tumor extracts will be presented. The use of MRS in discovering and monitoring novel molecular therapies, and in translating them into the clinic will also be discussed. Ex vivo MRS of cells and tumors provides opportunities to understand the role of metabolism in cancer immune surveillance and immunotherapy. 1H MR spectra obtained from breast cancer cells MD-MB-231 after siRNA treatment targeting either choline kinase or program death ligand 1 (PDL1) are shown in Figure 1 [1], and showed that downregulating PD-L1 resulted in significant changes in several metabolites that could be detected by 1H MRS. Applications of high-resolution MRS to characterize biofluid metabolic composition will be presented. Biofluids represent an easily accessible resource for metabolic phenotyping of disease processes. In cancer, biofluid metabolites are a composite reflecting the altered metabolism of the cancer as well as changes in organ metabolism induced by the cancer. The most frequently analyzed biofluids are plasma or serum, and urine. Interstitial fluid from cancers or ascites fluid can also be analyzed. Representative spectra acquired on ascitic fluid of 2 different ovarian cancer mice models are shown in Figure 2 [2]. With advances in computational capabilities, the integration of artificial intelligence to identify differences in multinuclear spectral patterns is providing exciting advances in detection and monitoring response to treatment. This lecture will overall outline the impact of MRS of extracted cells, tissues and biofluids, in the discovery, detection and treatment of cancer, and provide a perspective on exciting new directions for the future. The lecture will be for radiologists and scientists interested in cancer metabolism, and in high resolution MRS techniques.
Acknowledgements
Funding from the Emerson CollectiveReferences
1. Pacheco-Torres, J., et al., The PD-L1 metabolic interactome intersects with choline metabolism and inflammation. Cancer Metab, 2021. 9(1): p. 10.
2. Bharti, S.K., et al., Metabolomic characterization of experimental ovarian cancer ascitic fluid. Metabolomics, 2017. 13.
Figures
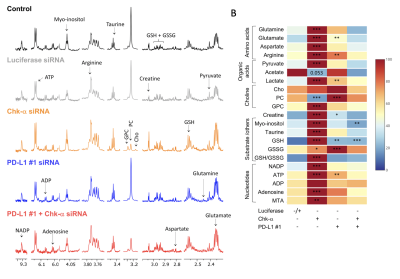
Figure 1: Representative 1H MR spectra obtained from MDA-MB 231 cells. Spectra are displayed from untreated cells (black), cells transfected with luciferase siRNA (light gray), with Chk-α siRNA (orange), with PD-L1 siRNA #1 (blue), and with a mixture of PD-L1 #1 and Chk-α siRNA (red). GPC, glycerophosphocholine; PC, phosphocholine; Cho, choline; GSH, glutathione; GSSG, oxidized glutathione; MTA, S-methyl-5′-thioadenosine. b Metabolic heat map, generated from quantitative analysis of high-resolution 1H MR spectral data [1].
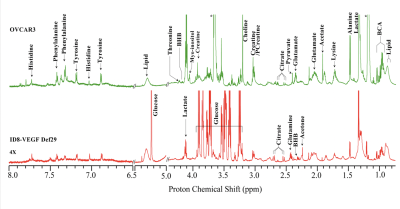
Figure 2: Representative CPMG 1H MR spectra obtained from ascitic fluid of an ID8-VEGF-Defb29 (Red), and an OVCAR3 (Green) tumor bearing mice. Expansions of the spectra from 6.5–8.0 ppm are 4X vertically zoomed. (BHB; betahydroxybutyrate, BCA; branch chain amino acid) [2]