MR Techniques for Quantifying Myocardial Perfusion
1Center of Marine Sciences - CCMAR, Faro, Portugal, 2School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom
Synopsis
Keywords: Contrast mechanisms: Perfusion, Cardiovascular: Cardiac, Image acquisition: Quantification
Myocardial perfusion imaging is an essential tool for characterising ischemic heart disease. Moreover, quantitative myocardial perfusion methods that provide pixel-wise quantitative myocardial perfusion maps are increasingly being applied as an alternative to visual inspection. Newer methods combine quantitative imaging with acceleration techniques and motion compensation to overcome current limitations of the technique, and thus, improve spatial resolution and heart coverage, reduce image degradation due to motion and accurately detect perfusion defects. In addition, fully automated workflows are facilitating the integration of quantitative myocardial perfusion into clinical practice by making it faster and easier to use.Introduction
First-pass perfusion cardiac MR (pCMR) enables the non-invasive detection of ischemic heart disease.1-3 This technique captures a series of images during the rapid passage of a contrast bolus through the heart. Acquisitions are electrocardiogram (ECG)-synchronised with the cardiac cycle and patients are instructed to hold their breath for as long as they can, to reduce image degradation due to cardiac and respiratory motion. Thus, conventional pCMR only permits the acquisition of 3-4 non-contiguous short-axis slices with moderate spatial resolution (~2.5mm). Moreover, diagnostic accuracy of pCMR may be compromised by respiratory motion artefacts because breath-holding can be challenging for patients, incomplete heart coverage, and dark-rim artefacts (false positives) due to low image resolution. In addition, images are often interpreted based on visual assessment, which only has high diagnostic accuracy when performed by highly trained operators.4 Recently, there has been a growing interest in developing automated pCMR methods that provide pixel-wise quantification of myocardial perfusion since they provide operator-independent, objective and reproducible results.3Technical Aspects
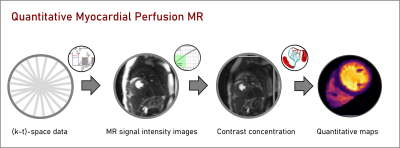
Quantitative imaging: Tracer kinetic modellingPerfusion quantification refers to the estimation of physiological parameters related to the microvascular environment (e.g., myocardial blood flow in ml/min/g, MBF) from the contrast enhancement obtained during the first pass of the contrast bolus, through the cardiac chambers and myocardium (Fig. 1).3 Quantitative imaging requires knowledge of the contrast agent concentration in the myocardial tissue and arterial input function (AIF; typically measured in the left ventricle blood pool). It is often assumed that there is a linear relationship between the MR signal intensities and the contrast agent concentration.
Quantitative pCMR has been performed using either the Fermi deconvolution method or (more complex) tracer-kinetic modelling.3,5,6 The Fermi method determines MBF through deconvolution of the AIF from the myocardial tissue concentration curve and by fitting an empirical-mathematical model. On the other hand, tracer-kinetic models are based upon physiological assumptions about the interaction between the contrast agent and different tissues and thus, can be used to extract several quantitative physiological parameters. However, the fitting problem can be less stable.
Sequences for quantitative pCMR: How to get data for accurate quantification of myocardial perfusion
The most commonly used pulse sequence for pCMR is a 2D saturation recovery pulse sequence. One major challenge with quantitative imaging is the lack of linearity between the MR signal intensity and the contrast agent concentration at high contrast agent concentrations. The concentration of contrast in the blood is much higher than in the myocardium, resulting in the saturation of the AIF.
The dual-bolus and dual-sequence methods have been proposed to handle the AIF saturation.7-11 The dual-bolus method uses a low dose bolus to measure the AIF followed by a high dose bolus for myocardial imaging. The dual-sequence method acquires a low-resolution slice with a short saturation-recovery time to estimate the AIF, together with high-resolution myocardial slices, without the need for additional contrast injection, but requires specialised CMR software.
Accelerated pCMR: Quantitative imaging with high resolution and/or heart coverage
Parallel imaging, compressed sensing and low-rank reconstruction methods have been proposed to accelerate 2D pCMR scans and achieve the necessary spatial resolution to eliminate dark-rim artefacts and improve diagnostic confidence.12-17 These methods explore the redundancies or compressibility of pCMR images to reduce the amount of (k-t)-space data required for image reconstruction. However, these methods still suffer from limited cardiac coverage. 2D simultaneous multi-slice sequences have been combined with undersampling techniques to improve both spatial resolution and coverage.18,19 3D pCMR with whole-heart coverage has been achieved using advanced k-t undersampling strategies without ECG-synchronisation and/or breath-holding.20-23 However, 3D pCMR methods often sacrifice spatial resolution.
Typically, acceleration methods indirectly generate quantitative myocardial perfusion maps by first reconstructing individual dynamic contrast-enhanced images, which are then converted to contrast agent concentration and, finally, tracer-kinetic modelling is used to generate quantitative maps (Fig.1). Recently, a model-based reconstruction method has been proposed to directly estimate quantitative perfusion maps and achieve extremely high acceleration rates.24,25
Deep learning-based methods have gained popularity in CMR due to their potential to significantly speed up reconstructions.26 Unfortunately, large amounts of fully sampled reference data are required for training these methods, which are not available in pCMR. Therefore, self-supervised deep learning pCMR methods have been proposed to reconstruct the dynamic pCMR times series from undersampled data without requiring fully sampled data.27-29
Automated workflows: Making quantitative pCMR easier to use and more accessible
Quantitative pCMR can be complex and time consuming, requiring manual segmentation and labelling of regions of interest in a large number of images. Automatic in-line vendor specific and vendor neutral commercial quantitative pCMR solutions have been proposed to enable fast, reproducible and operator-independent estimates of myocardial perfusion.11,30 These methods include respiratory motion correction, automatic detection of the AIF, segmentation and pixel-wise estimation of perfusion maps.
An in-line dual-saturation multi-echo Dixon pCMR framework (FOSTERS), with low-rank and sparsity constrained reconstruction, has been proposed to facilitate free-breathing and high-resolution pCMR.31 It automatically estimates non-rigid respiratory motion from fat-only images and generates high-resolution pixel-wise perfusion maps from motion-corrected water-only images.
Artificial intelligence-based solutions have also been proposed to automate the time-consuming and subjective pre-processing step of quantitative pCMR, such as segmentation and identification of the AIF.32-34
Conclusions
Quantitative pCMR is an established non-invasive test for the detection of ischemic heart disease. However, accurate quantification requires time-consuming manual processing steps and expert knowledge. This has prevented the widespread clinical adoption of quantitative pCMR. Recently, fully automated quantitative pCMR methods have been proposed to provide operator-independent, accurate and reproducible results in a faster and simpler way. These methods automate several tasks, such as reconstruction, motion correction, segmentation, AIF estimation and pixel-wise estimation of myocardial perfusion maps. Automated qCMR methods can also be combined with acceleration techniques to increase the spatial resolution and/or heart coverage, to improve the detection of perfusion defects.Acknowledgements
Funding sources: “la Caixa” Foundation and FCT, I.P. under the project code [LCF/PR/HR22/00533]; FCT through projects projects UIDB/04326/2020, UIDP/04326/2020 and LA/P/0101/2020.References
1. Ismail TF, Strugnell W, Coletti C, et al. Cardiac MR: From Theory to Practice. Front Cardiovasc Med 2022;9:826283.
2. Sharrack N, Chiribiri A, Schwitter J, Plein S. How to do quantitative myocardial perfusion cardiovascular magnetic resonance. Eur Heart J Cardiovasc Imaging 2022;23(3):315-318.
3. Jerosch-Herold M. Quantification of myocardial perfusion by cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2010;12(1):57.
4. Villa ADM, Corsinovi L, Ntalas I, et al. Importance of operator training and rest perfusion on the diagnostic accuracy of stress perfusion cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2018;20(1):74.
5. Scannell CM, Chiribiri A, Villa ADM, Breeuwer M, Lee J. Hierarchical Bayesian myocardial perfusion quantification. Med Image Anal 2020;60:101611.
6. Schwab F, Ingrisch M, Marcus R, et al. Tracer kinetic modeling in myocardial perfusion quantification using MRI. Magn Reson Med 2015;73(3):1206-1215.
7. Ishida M, Schuster A, Morton G, et al. Development of a universal dual-bolus injection scheme for the quantitative assessment of myocardial perfusion cardiovascular magnetic resonance. Journal of Cardiovascular Magnetic Resonance 2011;13.
8. Hsu LY, Rhoads KL, Holly JE, Kellman P, Aletras AH, Arai AE. Quantitative myocardial perfusion analysis with a dual-bolus contrast-enhanced first-pass MRI technique in humans. J Magn Reson Imaging 2006;23(3):315-322.
9. Gatehouse PD, Elkington AG, Ablitt NA, Yang GZ, Pennell DJ, Firmin DN. Accurate assessment of the arterial input function during high-dose myocardial perfusion cardiovascular magnetic resonance. J Magn Reson Imaging 2004;20(1):39-45.
10. Sanchez-Gonzalez J, Fernandez-Jimenez R, Nothnagel ND, Lopez-Martin G, Fuster V, Ibanez B. Optimization of dual-saturation single bolus acquisition for quantitative cardiac perfusion and myocardial blood flow maps. J Cardiovasc Magn Reson 2015;17(1):21.
11. Kellman P, Hansen MS, Nielles-Vallespin S, et al. Myocardial perfusion cardiovascular magnetic resonance: optimized dual sequence and reconstruction for quantification. J Cardiovasc Magn Reson 2017;19(1):43.
12. Tsao J, Boesiger P, Pruessmann KP. k-t BLAST and k-t SENSE: dynamic MRI with high frame rate exploiting spatiotemporal correlations. Magn Reson Med 2003;50(5):1031-1042.
13. Pedersen H, Kozerke S, Ringgaard S, Nehrke K, Kim WY. k-t PCA: temporally constrained k-t BLAST reconstruction using principal component analysis. Magn Reson Med 2009;62(3):706-716.
14. Otazo R, Candes E, Sodickson DK. Low-rank plus sparse matrix decomposition for accelerated dynamic MRI with separation of background and dynamic components. Magn Reson Med 2015;73(3):1125-1136.
15. Tourais J, Schneider T, Milidonis X, et al. High-Resolution motion-corrected 2D Myocardial Perfusion MRI using Locally Low Rank and Wavelet Sparsity Constraints. International Society for Magnetic Resonance in Medicine. Paris, France; 2019. p. 1238.
16. Scannell C, Schneider T, Alskaf E, et al. Free-Breathing High-Resolution Quantitative First-Pass Perfusion Cardiac MR using Dual-Echo Dixon. International Society for Magnetic Resonance in Medicine; 2021. p. 998.
17. Chen X, Salerno M, Yang Y, Epstein FH. Motion-compensated compressed sensing for dynamic contrast-enhanced MRI using regional spatiotemporal sparsity and region tracking: block low-rank sparsity with motion-guidance (BLOSM). Magn Reson Med 2014;72(4):1028-1038.
18. Sun C, Robinson A, Wang Y, et al. A Slice-Low-Rank Plus Sparse (slice-L + S) Reconstruction Method for k-t Undersampled Multiband First-Pass Myocardial Perfusion MRI. Magn Reson Med 2022;88(3):1140-1155.
19. McElroy S, Ferrazzi G, Nazir MS, et al. Combined simultaneous multislice bSSFP and compressed sensing for first-pass myocardial perfusion at 1.5 T with high spatial resolution and coverage. Magn Reson Med 2020;84(6):3103-3116.
20. Hoh T, Vishnevskiy V, Polacin M, Manka R, Fuetterer M, Kozerke S. Free-breathing motion-informed locally low-rank quantitative 3D myocardial perfusion imaging. Magn Reson Med 2022;88(4):1575-1591.
21. Fair MJ, Gatehouse PD, DiBella EV, Firmin DN. A review of 3D first-pass, whole-heart, myocardial perfusion cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2015;17:68.
22. Sharif B, Dharmakumar R, Arsanjani R, et al. Non-ECG-gated myocardial perfusion MRI using continuous magnetization-driven radial sampling. Magn Reson Med 2014;72(6):1620-1628.
23. Christodoulou AG, Shaw JL, Nguyen C, et al. Magnetic resonance multitasking for motion-resolved quantitative cardiovascular imaging. Nat Biomed Eng 2018;2(4):215-226.
24. Correia T, Schneider T, Chiribiri A. Model-Based Reconstruction for Highly Accelerated First-Pass Perfusion Cardiac MRI. Medical Image Computing and Computer Assisted Intervention – MICCAI 2019, Lecture Notes in Computer Science; 2019. p. 514-522.
25. Correia T, Schneider T, Chiribiri A. The key to extremely accelerated model-based quantitative first-pass perfusion cardiac MRI. International Society for Magnetic Resonance in Medicine; 2021. p. 508.
26. Leiner T, Rueckert D, Suinesiaputra A, et al. Machine learning in cardiovascular magnetic resonance: basic concepts and applications. J Cardiovasc Magn Reson 2019;21(1):61.
27. Martin-Gonzalez E, Alskaf E, Chiribiri A, et al. Physics-Informed Self-supervised Deep Learning Reconstruction for Accelerated First-Pass Perfusion Cardiac MRI. Lect Notes Comput Sc 2021;12964:86-95.
28. Martin-Gonzalez E, Alskaf E, Chiribiri A, et al. The deep SECRET to accelerated first-pass perfusion cardiac MRI. International Society for Magnetic Resonance in Medicine; 2022. p. 299.
29. Demirel OB, Yaman B, Shenoy C, Moeller S, Weingartner S, Akcakaya M. Signal intensity informed multi-coil encoding operator for physics-guided deep learning reconstruction of highly accelerated myocardial perfusion CMR. Magn Reson Med 2023;89(1):308-321.
30. Hsu LY, Jacobs M, Benovoy M, et al. Diagnostic Performance of Fully Automated Pixel-Wise Quantitative Myocardial Perfusion Imaging by Cardiovascular Magnetic Resonance. JACC Cardiovasc Imaging 2018;11(5):697-707.
31. Tourais J, Scannell CM, Schneider T, et al. High-Resolution Free-Breathing Quantitative First-Pass Perfusion Cardiac MR Using Dual-Echo Dixon With Spatio-Temporal Acceleration. Front Cardiovasc Med 2022;9:884221.
32. Xue H, Davies RH, Brown LAE, et al. Automated Inline Analysis of Myocardial Perfusion MRI with Deep Learning. Radiol Artif Intell 2020;2(6):e200009.
33. Scannell CM, Alskaf E, Sharrack N, et al. AI-AIF: artificial intelligence-based arterial input function for quantitative stress perfusion cardiac magnetic resonance. Eur Heart J Digit Health 2023;4(1):12-21.
34. Scannell CM, Veta M, Villa ADM, et al. Deep-Learning-Based Preprocessing for Quantitative Myocardial Perfusion MRI. J Magn Reson Imaging 2020;51(6):1689-1696.