Blood-Brain Barrier Dysfunction in Neurological Diseases
1University of Southern California (USC), United States
Synopsis
Keywords: Neuro: Brain function, Contrast mechanisms: Perfusion, Image acquisition: Quantification
Blood-brain barrier (BBB) plays a critical role in the delivery of oxygen and nutrients to the brain, clearance of toxic metabolites, and protection of the central nervous system (CNS) from infection. Impaired BBB function is implicated in a number of serious CNS diseases, such as multiple sclerosis, stroke, brain tumors, Alzheimer's disease and small vessel disease. Assessment of BBB permeability commonly relies on dynamic contrast enhanced MRI using Gd-based contrast agents. Recently, contrast and non-contrast MRI methods have emerged to assess BBB water exchange. The combination of these MRI methods has revealed the mechanism of BBB dysfunction in neurological disorders.Introduction
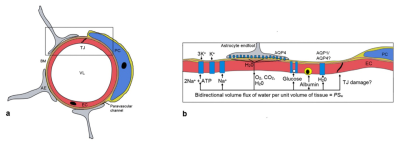
Blood-brain barrier (BBB) plays a critical role in the delivery of oxygen and nutrients to the brain, clearance of toxic metabolites, and protection of the central nervous system (CNS) from infection. Compromised or impaired BBB function is implicated in a number of serious CNS diseases, such as multiple sclerosis (MS), stroke, brain tumors, CNS infection, Alzheimer's disease (AD) and cerebral small vessel disease (cSVD). As shown in Fig. 1, the BBB consists of several functional elements (1): endothelial cells (EC) lining the capillaries with tight junctions (TJs) between ECs. The endothelial monolayer is surrounded by a discontinuous layer of pericytes separated by the basement membrane. Adjacent to the pericytes are the astrocyte feet on which aquarporin-4 (AQP4) water channels are distributed. Damage to the BBB can increase the permeability of the walls of the blood vessels within the brain, leading to the influx of neurotoxic and pro-inflammatory molecules, and invoking local inflammatory responses. This, in turn, further disrupts the integrity of the BBB and may lead to hemorrhagic transformation in stroke, metastatic initiation in tumors, or neurodegeneration in AD.Methods
Currently, assessment of BBB permeability relies on CSF sampling of albumin through lumbar puncture to measure CSF/plasma albumin ratio and/or dynamic contrast enhanced (DCE) MRI using Gd-based contrast agents (GBCAs). Since albumin (66 kDa) and GBCAs (~550 Da) have relatively large molecular weights, BBB permeability has to reach a critical level before extravasation occurs. In addition, GBCAs have potential renal complications, and have been linked to Gd deposition in the brain especially in persons undergoing repeated DCE scans. Recently, both contrast and non-contrast MRI methods have emerged to assess BBB water exchange (1). In contrast agent based approaches, an intravascular contrast agent is introduced into the blood pool to increase intravascular-extravascular T1 differences. Because T1 interactions are short range, any changes in the T1 of the extravascular compartment can be attributed to trans-BBB water exchange. In contrast agent free approaches, a number of ASL methods have been introduced for assessment of BBB function, including diffusion-weighted (DW) ASL and multi-echo (ME) ASL techniques for measuring the rate of water exchange (kw) across the BBB (2-5).Results
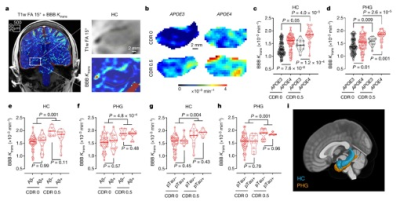
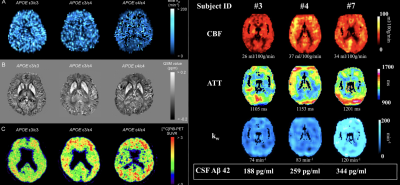
While DCE MRI measures of Ktrans are sensitive to BBB disruption in tumor, stroke and MS, it remains challenging to measure subtle changes of BBB permeability in aging, AD and cSVD, which requires long scan time and there are considerable heterogeneities in imaging parameters and protocols (6). Community efforts for harmonizing DCE MRI protocols have emerged such as HARNESS consortium (7). Existing evidence showed Ktrans in hippocampus increases in APOE4 carriers and subjects with mild cognitive impairment (MCI)(8,9) (Fig. 2). However, Ktrans was not associated with Abeta or tau protein. There is also converging evidence supporting increased BBB permeability in cSVD (10). However, recent studies using DW ASL reported reduced BBB water exchange rate kw in aging, cognitive decline and cSVD (11-14), and decreased kw was associated with Abeta in CSF or PET (Fig. 3). These seemingly contradictive findings between BBB permeability to GBCA and water exchange rate can be explained in the context of multiple components of the BBB and their different functions. In fact, contrast-based MRI studies have reported increased Ktrans and reduced kw in aging, MS and brain tumor (15,16), which has been attributed to active co-transport of water across the BBB through Na,K-ATPase pump.Summary and Discussion
Accumulating evidence suggests that BBB dysfunction is a core mechanism in a number of neurological disorders. Emerging contrast and noncontrast MRI methods have been developed to assess BBB function and helped unveil the underlying biological mechanisms of BBB dysfunction and failure in a range of neurologic disorders.Acknowledgements
NIH grants UF1-NS100614, R01-EB014922 and RF1-NS122028References
1. Dickie BR, Parker GJM, Parkes LM. Measuring water exchange across the blood-brain barrier using MRI. Prog Nucl Magn Reson Spectrosc 2020;116:19-39.
2. Wang J, Fernandez-Seara MA, Wang S, St Lawrence KS. When perfusion meets diffusion: in vivo measurement of water permeability in human brain. J Cereb Blood Flow Metab 2007;27(4):839-849.
3. St Lawrence KS, Owen D, Wang DJ. A two-stage approach for measuring vascular water exchange and arterial transit time by diffusion-weighted perfusion MRI. Magn Reson Med 2012;67(5):1275-1284.
4. Shao X, Ma SJ, Casey M, D'Orazio L, Ringman JM, Wang DJJ. Mapping water exchange across the blood-brain barrier using 3D diffusion-prepared arterial spin labeled perfusion MRI. Magn Reson Med 2019;81(5):3065-3079.
5. Gregori J, Schuff N, Kern R, Günther M. T2-based arterial spin labeling measurements of blood to tissue water transfer in human brain. J Magn Reson Imaging 2013;37(2):332-342.
6. Raja R, Rosenberg GA, Caprihan A. MRI measurements of Blood-Brain Barrier function in dementia: A review of recent studies. Neuropharmacology 2018;134(Pt B):259-271.
7. Thrippleton MJ, Backes WH, Sourbron S, Ingrisch M, van Osch MJP, Dichgans M, Fazekas F, Ropele S, Frayne R, van Oostenbrugge RJ, Smith EE, Wardlaw JM. Quantifying blood-brain barrier leakage in small vessel disease: Review and consensus recommendations. Alzheimers Dement 2019;15(6):840-858.
8. Montagne A, Barnes SR, Sweeney MD, Halliday MR, Sagare AP, Zhao Z, Toga AW, Jacobs RE, Liu CY, Amezcua L, Harrington MG, Chui HC, Law M, Zlokovic BV. Blood-brain barrier breakdown in the aging human hippocampus. Neuron 2015;85(2):296-302.
9. Montagne A, Nation DA, Sagare AP, Barisano G, Sweeney MD, Chakhoyan A, Pachicano M, Joe E, Nelson AR, D'Orazio LM, Buennagel DP, Harrington MG, Benzinger TLS, Fagan AM, Ringman JM, Schneider LS, Morris JC, Reiman EM, Caselli RJ, Chui HC, Tcw J, Chen Y, Pa J, Conti PS, Law M, Toga AW, Zlokovic BV. APOE4 leads to blood-brain barrier dysfunction predicting cognitive decline. Nature 2020;581(7806):71-76.
10. Farrall AJ, Wardlaw JM. Blood-brain barrier: ageing and microvascular disease--systematic review and meta-analysis. Neurobiol Aging 2009;30(3):337-352.
11. Gold BT, Shao X, Sudduth TL, Jicha GA, Wilcock DM, Seago ER, Wang DJ. Water exchange rate across the blood-brain barrier is associated with CSF amyloid-beta42 and cognitive performance in healthy older adults. Alzheimer's and Dementia 2021:(In press).
12. Li Y, Ying Y, Yao T, Jia X, Liang H, Tang W, Song H, Shao X, Wang DJJ, Wang C, Cheng X, Yang Q. Decreased water exchange rate across blood-brain barrier in hereditary cerebral small vessel disease. Brain 2023.
13. Ford JN, Zhang Q, Sweeney EM, Merkler AE, de Leon MJ, Gupta A, Nguyen TD, Ivanidze J. Quantitative Water Permeability Mapping of Blood-Brain-Barrier Dysfunction in Aging. Front Aging Neurosci 2022;14:867452.
14. Uchida Y, Kan H, Sakurai K, Horimoto Y, Hayashi E, Iida A, Okamura N, Oishi K, Matsukawa N. dose associates with increased brain iron and β-amyloid via blood-brain barrier dysfunction. J Neurol Neurosurg Psychiatry 2022.
15. Rooney WD, Li X, Sammi MK, Bourdette DN, Neuwelt EA, Springer CS. Mapping human brain capillary water lifetime: high-resolution metabolic neuroimaging. NMR Biomed 2015;28(6):607-623.
16. Anderson VC, Tagge IJ, Li X, Quinn JF, Kaye JA, Bourdette DN, Spain RI, Riccelli LP, Sammi MK, Springer CS, Rooney WD. Observation of Reduced Homeostatic Metabolic Activity and/or Coupling in White Matter Aging. J Neuroimaging 2020;30(5):658-665.
Figures