Clinical Trials & Practice: MRI: Present & Future
1Normandie Univ. UNICAEN, Caen, France
Synopsis
Keywords: Contrast mechanisms: Molecular Imaging
MRI of neuroinflammation currently relies on two main imaging features: edema and blood brain barrier leakage. Unfortunately, they are not specific for inflammation and the diagnosis of inflammatory lesions of the central nervous system can be challenging. New imaging biomarkers have been described such as the paramagnetic rim sign in multiple sclerosis, that improve imaging specificity but remain only applicable to a subset of neuroinflammatory diseases. More recently, there have been striking progresses in preclinical molecular MRI including the development of highly sensitive and specific contrast agents targeting the inflamed neurovascular unit with potential for clinical translation.
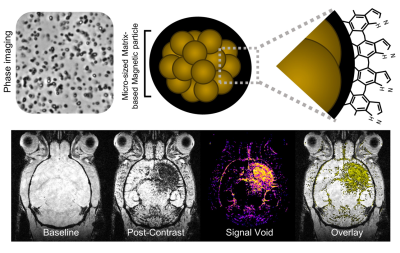
The main limitation of MRI is its low sensitivity to detect exogenous contrast agent, necessitating amplification strategies for molecular imaging (Gauberti, Fournier et al. 2018). Indeed, most relevant targets for molecular imaging have concentrations in the range 10-9 – 10-12 M in human tissues, whereas MRI detects clinically approved gadolinium chelate at concentrations over 10-6 M. It is thus necessary to bind at least 103 to 106 contrast producing atoms per molecular target to reach a sensitivity high enough for detection by MRI. Early attempts of molecular MRI of the central nervous system were aimed at revealing parenchymal proteins or cells (de Backer, Nabuurs et al. 2010). However, crossing of the blood brain barrier in concentration high enough to produce contrast on MRI is still an unsolved challenge in the field. Both receptor-mediated and receptor-independent pathways to reach the brain parenchyma are theoretically possible (Kucharz, Kristensen et al. 2021, Li, Zheng et al. 2021), but to date, only proof of concept experiments in animal models involving blood brain barrier leakage have been described. For instance, Hubert et al. recently reported in a mouse stroke model that a nanoprobe (NanoGd) accumulates within the ischemic lesion after intravenous injection and is caught in situ by immune phagocytic cells (microglia and infiltrated monocytes) (Hubert, Hristovska et al. 2021), which could be used as a biomarker of neuroinflammation. However, as a general drawback of contrast agents requiring to cross the blood brain barrier to reach their target, their accumulation in the brain is heavily dependent on the permeability of the blood brain barrier (Gauberti, Montagne et al. 2014, Gauberti, Fournier et al. 2018). For instance, in the absence of blood brain barrier leakage, they will not induce any signal change, even in the presence of intense parenchymal phagocytic activity. Thus, in most cases, they offer only limited information on the presence of their target.
To solve this issue, recent studies on molecular MRI of neuroinflammation focused on targeting the endothelial cells of the neurovascular unit. The close interconnections between the brain parenchyma and the cerebrovasculature make the endothelial cell an interesting target for molecular imaging (Wu, Liu et al. 2017). In the context of neuroinflammation, phenotypic changes occur in endothelial cells, leading to the overexpression of specific proteins that can be used for molecular imaging. The most striking advances in the field have been achieved using contrast agents targeting adhesion molecules on activated endothelial cells (Gauberti, Fournier et al. 2018). In this presentation, we will describe the present status of MRI of neuroinflammation in clinical practice, the basic principles of molecular MRI of neuroinflammation, the most recent advances in the field and the remaining challenges in the production of clinically compatible contrast agents.
Acknowledgements
MG is funded by the “Fondation Bettencourt Schueller” for a CCA-Inserm-Bettencourt position. This This work was supported by the ANR “MAD-GUT” (ANR-19-CE19-0018) and “FLAMRING” (ANR-20-CE19-0032-01). MG is author on two patent for the use of biodegradable MPIO as contrast agent for molecular imaging.References
Gauberti, M., A. P. Fournier, F. Docagne, D. Vivien and S. Martinez de Lizarrondo (2018). "Molecular Magnetic Resonance Imaging of Endothelial Activation in the Central Nervous System." Theranostics 8(5): 1195-1212.
Gauberti, M., A. Montagne, O. A. Marcos-Contreras, A. Le Béhot, E. Maubert and D. Vivien (2013). "Ultra-sensitive molecular MRI of vascular cell adhesion molecule-1 reveals a dynamic inflammatory penumbra after strokes." Stroke 44(7): 1988-1996.
Hubert, V., I. Hristovska, S. Karpati, S. Benkeder, A. Dey, C. Dumot, C. Amaz, N. Chounlamountri, C. Watrin, J. C. Comte, F. Chauveau, E. Brun, P. Marche, F. Lerouge, S. Parola, Y. Berthezène, T. Vorup-Jensen, O. Pascual and M. Wiart (2021). "Multimodal Imaging with NanoGd Reveals Spatiotemporal Features of Neuroinflammation after Experimental Stroke." Adv Sci (Weinh): e2101433.
Kucharz, K., K. Kristensen, K. B. Johnsen, M. A. Lund, M. Lønstrup, T. Moos, T. L. Andresen and M. J. Lauritzen (2021). "Post-capillary venules are the key locus for transcytosis-mediated brain delivery of therapeutic nanoparticles." Nat Commun 12(1): 4121.
Li, J., M. Zheng, O. Shimoni, W. A. Banks, A. I. Bush, J. R. Gamble and B. Shi (2021). "Development of Novel Therapeutics Targeting the Blood-Brain Barrier: From Barrier to Carrier." Adv Sci (Weinh): e2101090.
Wu, F., L. Liu and H. Zhou (2017). "Endothelial cell activation in central nervous system inflammation." J Leukoc Biol 101(5): 1119-1132.