5434
Efficient fat suppression and motion correction using a Dixon PROPELLER sequence with interleaved echoes and asymmetric readout waveforms1Karolinska University Hospital, Stockholm, Sweden, 2Karolinska Institutet, Stockholm, Sweden, 3Clinical Neuroscience, Karolinska Institutet, Stockholm, Sweden
Synopsis
A novel fat/water separated Propeller Dixon sequence is described. Its performance is tested against fatsat.
Background
Accurate diagnosis and characterization of orbital and sinonasal pathologies are challenging due to field inhomogeneities and the presence of motion artifacts. A sequence with uniform fat suppression and reduced sensitivity to motion artifacts are undeniably essential in this anatomical region.The standard fat saturation method (1) is centered around a single fat frequency and can not account for the complex spectrum of fat with multiple resonances and off resonance frequencies due to field inhomogeneities, resulting in incomplete fat saturation.
Dixon sequences have a different approach of handling the fat signal by aligning echoes to acquire specific chemical shift encodes (CSE), where the fat signal is separated through a model based reconstruction without any signal destruction. Acquiring the additional encodes in CPMG sequences (e.g. FSE or PROPELLER) requires dead time between refocusing pulses, prolonging scan time and echo spacing. A double echo Dixon sequence therefore typically takes more than twice as long to acquire. Recently, we proposed a way to overcome the scan time penalty with asymmetric readouts in an FSE sequence(2).
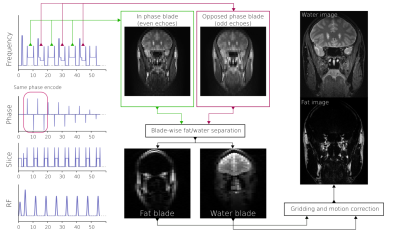
PROPELLER is a motion insensitive sequence due to its ability to average measurements continuously throughout acquisition, as well as allowing rigid body motion correction between blades(3). The combination of Dixon and PROPELLER however, is not straightforward, as most variants of the Dixon method require at least two echoes. Sampling each blade twice: first with water and fat in phase, then again with water and fat out of phase, results in a low temporal resolution where motion artifacts are amplified. Instead, acquiring the echoes interleaved where two blades are acquired in one shot, allows Dixon fat/water separation on blade pairs, reducing the temporal resolution to milliseconds rather than minutes.
This work describes a new T2-weighted Dixon PROPELLER pulse sequence with asymmetric readouts acquired with interleaved echoes. The abstract describes the implementation, as well as investigates its performance on still and moving healthy volunteers in coronal orbital and sinonasal scans. Comparisons are made with T2 FSE Dixon, and fatsat PROPELLER.
Methods
A T2 PROPELLER Dixon sequence was implemented in-house with asymmetric readout waveforms (2). Chemical shift encodes were acquired in an interleaved manner, i.e. the same phase encode was acquired twice with different CSE(4), shown in Figure 1. The sequence was compared against spectral fat-saturated PROPELLER to compare fat suppression performance. An FSE Dixon sequence was also included for comparison. The FSE Dixon sequence acquired in-phase and opposed phase echoes sequentially.Two healthy volunteers were enrolled in the study and gave written informed consent according to the institutional review board. First volunteer was instructed not to move to generate non-corrupted images from each sequence. The second volunteer was instructed to deliberately imitate an involuntary movement a patient might be expected to make: intermittently blinking and swallowing, breathing heavily, and moving the head.
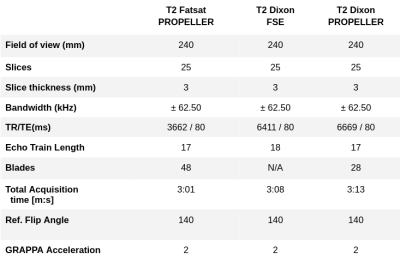
Coronal images were planned orthogonal to optic nerves covering orbito-nasal structures where the strong field inhomogeneities are expected to cause signal drop out in case of erronous fat saturation. Data was acquired on a GE Signa Premier 3T system with a 48-channel receive head coil. The scan parameters are summarized in Table 1. Images were reconstructed using an in-house developed PROPELLER reconstruction using the FWQPBO fat/water algorithm(5) with multi-peak fat spectrum modelling(6) and real-valued estimates (7).
Results
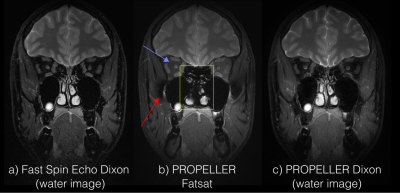
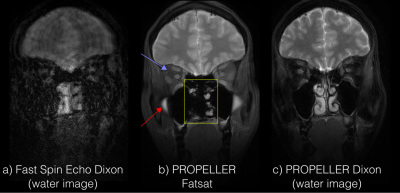
Figure 3 shows the comparison between Dixon FSE, fatsat PROPELLER, and the proposed Dixon PROPELLER sequence. Non-uniform fat suppression is seen with fatsat in areas with large field inhomogeneities, which is accounted for in the Dixon reconstruction. Ethmoid sinus is erroneously suppressed with fatsat.Figure 4 shows images acquired on a moving volunteer. Significant motion artifacts are present in the FSE images, rendering them nondiagnostic. Retrospective motion correction successfully corrects for this, with some loss in image sharpness.
Conclusion
The multi-peak fat model in Dixon results in a stronger fat suppression compared to fatsat, which shows residual fat signal surrounding the optic nerve (blue arrows, Figure 2-3). Our study has shown that this improved image quality can be achieved without scan time penalties using asymmetric readout waveforms.Residual fat signal and water suppression artifacts, in this case, completely changed their character (red arrow) between the subjects. This study shows that PROPELLER Dixon can account for motion correction and still maintain proper fat suppression, while FSE produces non-diagnostic images. Dixon PROPELLER is the only sequence able to depict the ethmoid sinuses. An improved image sharpness is seen in FSE Dixon compared to PROPELLER, which is expected from the Cartesian sampling.
The combination of complete fat-suppression with effective motion insensitive PROPELLER sampling is therefore a preferable choice of sequence in the sinonasal and orbital region. Considering the promising results in this study it would be interesting to test its feasibility in abdominal imaging as well as deep spaces in the head and neck area.
Acknowledgements
No acknowledgement found.References
1. Haase A, Frahm J, Hänicke W, Matthaei D. 1H NMR chemical shift selective (CHESS) imaging. Phys. Med. Biol. 1985;30:341–344.
2. Rydén H, Norbeck O, Avventi E, et al. Chemical shift encoding using asymmetric readout waveforms. Magn. Reson. Med. 2020;42:963.
3. Pipe JG. Motion correction with PROPELLER MRI: application to head motion and free-breathing cardiac imaging. Magn. Reson. Med. 1999;42:963–969.
4. Schär M, Eggers H, Zwart NR, Chang Y, Bakhru A, Pipe JG. Dixon water-fat separation in PROPELLER MRI acquired with two interleaved echoes. Magn. Reson. Med. 2016;75:718–728.
5. Berglund J, Skorpil M. Multi-scale graph-cut algorithm for efficient water-fat separation. Magn. Reson. Med. 2017;78:941–949.
6. Yu H, Shimakawa A, McKenzie CA, Brodsky E, Brittain JH, Reeder SB. Multiecho water-fat separation and simultaneous R2* estimation with multifrequency fat spectrum modeling. Magn. Reson. Med. 2008;60:1122–1134.
7. Berglund J, Rydén H, Avventi E, Norbeck O, Sprenger T, Skare S. Fat/water separation in k-space with real-valued estimates and its combination with POCS. Magn. Reson. Med. 2020;83:653–661.
Figures