5433
MR-safety of mixed-brand of cardiac implantable electronic devices: Comparison of RF induced heating with approved single-brand at 1.5 T and 3.0 T1Department of Radiological Technology, Juntendo University, Tokyo, Japan, 2BioView, Inc., Tokyo, Japan, 3Department of Medicine, Division of Cardiology, Nihon University School of Medicine, Tokyo, Japan, 4Department of Medicine, University of Occupational and Environmental Health, Fukuoka, Japan, 5Department of Radiology, Juntendo University Hospital, Tokyo, Japan, 6Division of Medical Devices, National Institute of Health Sciences, Kanagawa, Japan, 7School of Information Science and Technology, Tokai University, Kanagawa, Japan
Synopsis
Radio-frequency-induced heating around MR-conditional cardiac implantable electronic devices with mixed brand combinations of generator and lead were compared with the approved single brand combinations at 1.5 T and 3.0 T. The generator-lead combinations were selected from three high-share vendors (Boston Scientific, Medtronic, and St. Jude) based on the frequency in clinical practice. Temperature measurements were conducted along with ASTM2182 and ISO10974 at locations corresponding to the right ventricle, right atrium, the generator edge, and contralateral of in the ASTM phantom. The results evaluated by Mann-Whitney's U-test showed no significant difference in temperature increases between the mixed and approved combinations.
Introduction
MR safety of patients who have implantable medical devices is essential, but the regulatory approvals for multiple devices are needed to consider further conditions, such as standards, technical specifications, and guidelines.1 MR-conditional cardiac implantable electric devices approved by the certification body lose their MR-conditionality when the generator is replaced with a product from a different brand than that of the lead. Previous reports showed that MRI in patients with different brands (mixed-brand) did not result in increased patient risk or significant device changes compared to same brands (single-brand).2,3 Other reports showed that the RF impedance of generators is minimal relative to that of the leads, which dominates the overall impedance of cardiac implantable electric devices. 4,5 The present study was performed to investigate the effect of such mixed-brand combinations in terms of RF-induced heating.Methods
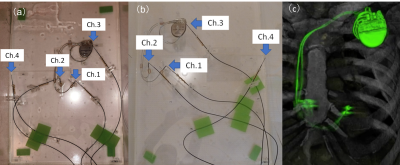
A pair of pacemaker leads of a vendor were connected with a generator of the single-brand and with generators of mixed-brand. Each combination was immersed in the ASTM phantom and scanned with 2D-FSE at 3.0 T (Skyra, Siemens) and 1.5 T (Philips, Ingenia). Imaging parameters at 3.0T were as follows: TR/TE, 7110/5.3 msec; Flip angle=180°, FOV=400×400, matrix=256×256, slice thickness=10 mm, number of slices=36, SAR=1.3 W/kg, B1+RMS=2.5 μT, scan time=17min and 5sec. Imaging parameters at 1.5T were as follows: TR/TE, 16910/3.7 msec, Flip angle=180°, FOV=400×400, matrix=252×250, slice thickness=10 mm, number of slices=28, SAR=2.6 W/kg, B1+RMS=4.31 μT, scan time=16minutes and 46seconds. Temperature was measured with optical thermometers at locations corresponding to the right ventricle (Ch1), right atrium (Ch2), the generator edge (Ch3), and contralateral of in the phantom (Ch4) as described in Figure 1. The three-dimensional trajectories of the leads were determined based on the X-ray CT images of typical clinical cases. The results were evaluated by net temperature, which were deducted by the results of no device. We used Boston Scientific, Medtronic, and St. Jude products (3 single-brand sets) and 5-6 mixed-brand sets. We compared the mixed-brand combinations and single-brand using Mann-Whitney's U-test.Results
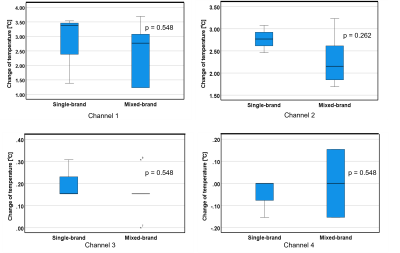
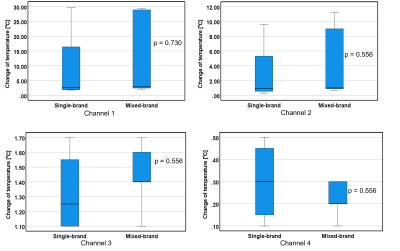
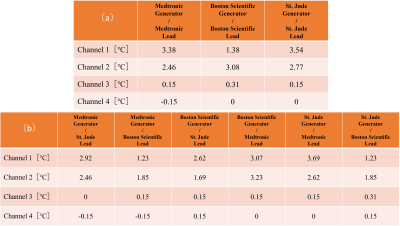
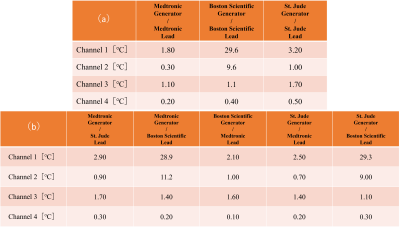
The results in Fig. 2-5 showed that there were no significant differences in temperature elevations between the single- and mixed-brand combinations. Note that there were remarkable temperature increases in the combinations of Boston Scientific lead at 1.5 T. Boston Scientific generator-lead was 29.6 ℃ (Ch1), 9.6 ℃ (Ch2), 1.1 ℃ (Ch3), 0.4 ℃ (Ch4). St. Jude generator and Boston Scientific lead were 29.3 ℃ (Ch1), 9.0 ℃ (Ch2), 1.1 ℃ (Ch3), 0.3 ℃ (Ch4). Medtronic generator and Boston Scientific lead were 28.9 ℃ (Ch1), 11.2 ℃ (Ch2), 1.4 ℃ (Ch3), 0.2 ℃ (Ch4). The repeatability of some combinations was assessed using St. Jude and Medtronic products at 1.5T. The average and standard deviation of three measurements using St. Jude generator and lead were 28.7±0.06 ℃ (Ch1), 1.03±0.15 ℃ (Ch2), 1.57±0.23 ℃ (Ch3), 0.30±0.10 ℃ (Ch4) , respectively. The average and standard deviation of three measurements using St. Jude generator and Medtronic lead were 27.7±0.21 ℃ (Ch1), 1.57±0.12 ℃ (Ch2), 1.50±0.20 ℃ (Ch3), 0.10±0.17 ℃ (Ch4) , respectively. The reproducibility of placement was also demonstrated using St. Jude generator and lead products at 3.0T. The results were 3.54 ℃ (Ch1), 2.77 ℃ (Ch2), 0.15 ℃ (Ch3), 0 ℃ (Ch4) in Figure 1 (a) setting and 2.36 ℃ (Ch1), 2.36 ℃ (Ch2), -0.22 ℃ (Ch3), -0.04 ℃ (Ch4) in Figure 1 (b) setting.Discussion
In this study, we examined the differences of RF-induced heating with mixed-brand and single-brand combinations at 3.0 T and 1.5 T. Although there were no significant differences between mixed-brand and single-brand combinations, our results showed that there were noticeable temperature elevations in the combinations of Boston Scientific lead at 1.5 T. Previous study showed that the RF impedance of leads is maximum relative to that of the generators, which dominates the overall impedance of cardiac implantable electric devices. 4,5 If the leads dominate the impedance of pacemaker, it is probable that RF induced heating also dominates that.Conclusion
Our study showed that there was no significant difference in temperature increase between the mixed and approved combinations. This study may serve as a base for the safety of mix-brand combinations during MRI scan. In the future, the influence of residual lead and epicardial leads will have to be examined.Acknowledgements
We thank Asami Masuda
for experiment preparation.
References
1. Kuroda K, Yatsushiro S. New Insights into MR Safety for Implantable Medical Devices.Magn Reson Med Sci 2022; 21: 110–131.
2. Minaskeian N, Hajnal SP, Luis Rp Scott, et al. Safety of magnetic resonance imaging in patients with cardiac implantable electronic devices with generator and lead(s) brand mismatch. J Appl Clin Med Phys. 2022 Mar;23(3):e13520.
3. König CA, Tinhofer F, and Zweiker D. Is diversity harmful?—Mixed-brand cardiac implantable electronic devices undergoingmagnetic resonance imaging. Wien Klin Wochenschr. 2022; 134: 286–293 4. Meyers J, Prutchi D, and Shehada R. Input Impedance Comparison of MR-Conditional Cardiac Implantable Pulse Generators at the 1.5T MR Frequency of 63.87 MHz. Proceeding of 30th Annual Meeting of ISMRM, Online, 2021; 2311.
5. Prutchi D, Meyers J, and Shehada R. RF Impedance of MR-Conditional Pacemaker Leads when Connected to Implantable Pulse Generators from Different MR-Conditional Systems. Proceeding of 30th Annual Meeting of ISMRM, Online, 2021; 2281.
Figures