5431
Prognostic role of right ventricular late gadolinium enhancement in patients with Tetralogy of Fallot undergoing pulmonary valve replacement1Università degli Studi di Milano, Milan, Italy, 2ASST Nord Milano, Milan, Italy, 3Università degli Studi di Milano - IRCCS Policlinico San Donato, Milano, Italy, 4Università degli Studi di Milano - IRCCS Policlinico San Donato, Milan, Italy
Synopsis
Our purpose was to evaluate the correlations between right ventricular (RV) late gadolinium enhancement (LGE) at cardiac magnetic resonance (CMR) in patients with Tetralogy of Fallot (ToF) scheduled for pulmonary valve replacement (PVR) and post-PVR functional data. After assessing a semiquantitative LGE scoring for the RV, we observed a correlation between such score and RV post-PVR outcomes appraised at CMR. The assessment of RV LGE before PVR may provide prognostic insights on post-PVR functional outcomes, potentially facilitating a patient-tailored treatment pathway.
Background
Tetralogy of Fallot (ToF) is the most common cyanotic heart defect, with an estimated prevalence of 3 cases every 10,000 live births [1]. Although valve-sparing techniques are encouraged for surgical repair, transannular patch is commonly needed for the relief of outflow obstruction, leading to free pulmonary regurgitation and chronic volume right ventricle (RV) overload [2]. Current guidelines indicate specific timings for pulmonary valve replacement (PVR) based on RV data, to revert ventricular remodelling and achieve the best outcomes [3]. Cardiac magnetic resonance (CMR) is a pivotal tool in the follow-up of this population, being the standard-of-care for the functional assessment of the RV [4]. Along with ventricular morphology and function, contrast enhanced CMR allows the assessment of focal myocardial fibrosis through late gadolinium enhancement (LGE). Therefore, the aim of the present study was to evaluate LGE in ToF patients undergoing PVR and to assess its potential correlations with post-PVR CMR data.Methods
We retrospectively included all repaired ToF patients who underwent at least two CMR examinations (CMR‑0 and CMR‑1) at our institution, and who underwent PVR during the intercurrent time interval.All patients underwent CMR examinations acquired on a 1.5‑T unit (Magnetom Aera, Siemens Healthineers, Erlangen, Germany) with a gradient power of 45‑mT/m. All examinations were performed using a 48‑channel surface phased‑array coil which was placed over patients’ chests while lying supine. Each acquisition included LGE sequences acquired after intravenous administration of 0.10 to 0.15 mmol/kg of gadobutrol (Gadovist, Bayer Healthcare, Leverkusen, Germany).
For each CMR examination, short-axis cine sequences were segmented as part of the usual clinical workflow, by a radiologist for the assessment of LV and RV indexed end-diastolic volume (EDVi), indexed end-systolic volume (ESVi), stroke volume (SV) and ejection fraction (EF).
The same radiologist reviewed all CMR datasets and scored all LGE sequences both according to the semiquantitative scoring system proposed by Babu-Narayan et al. [5], and quantitatively by segmenting short-axis LGE stacks.
Results
Out of 294 individual patients with ToF who underwent CMR at our institution, 48 had CMR examinations performed both before and after PVR, and, among them, 15 had undergone contrast enhanced CMR before PVR. Hence, our final study population was composed by 15 patients who underwent PVR between two consecutive CMR examinations, 9 (60%) of whom males, with a median age of 25 years (IQR 16−29 years), acquired at a median interval of 17 months (IQR 12−23 months). The median interval between CMR-0 and PVR was 6 months (IQR 0−11 months), whereas the median interval between PVR and CMR-1 was 7 months (IQR 7−10 months).Between CMR-0 and CMR-1, LV EDVi increased significantly (p=0.025) from 70 mL/m2 (IQR 61−72 mL/m2) to 81 mL/m2 (IQR 69−92 mL/m2) and LV ESVi increased with borderline significance (p=0.050) from 24 mL/m2 (IQR 21−28 mL/m2) to 32 mL/m2 (IQR 25−35 mL/m2), while RV EDVi decreased significantly (p=0.009) from 120 mL/m2 (IQR 100−128 mL/m2) to 94 mL/m2 (IQR 82−101 mL/m2), and RV ESVi decreased significantly (p=0.048) from 53 mL/m2 (IQR 41−61 mL/m2) to 42 mL/m2 (IQR 34−48 mL/m2.
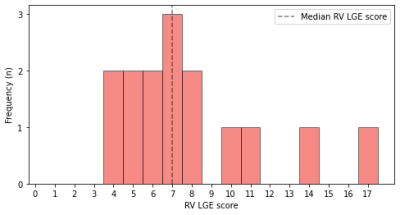
All 15 patients presented some degree of LGE. The median RV LGE score of our patients at CMR-0 was 7 (IQR 6−9), while the median absolute extent of LGE was 4.49 mL (IQR 3.70−5.78 mL), accounting for 5.63% (IQR 4.92−7.00%) of the RV volume. While LGE extent correlated with LGE percentage (ρ=0.550, p=0.034), there were no significant correlations between RV LGE score and LGE extent (ρ=0.099, p=0.726) or percentage (ρ=0.273, p=0.324). Moreover, RV LGE score displayed a strong negative correlation with RV SV (ρ=-0.609, p=0.016).
Conclusions
The main findings of this study include the correlations between pre-procedural RV scarring in patients referred for PVR and post-PVR variations of functional cardiac parameters, suggest that a higher extent of myocardial scar might be a predictor of worse ventricular remodelling.Acknowledgements
No acknowledgement found.References
1. Bailliard F, Anderson RH (2009) Tetralogy of Fallot. Orphanet J Rare Dis 4:2. doi:10.1186/1750-1172-4-2.
2. Puranik R, Tsang V, Lurz P, et al (2012) Long-term importance of right ventricular outflow tract patch function in patients with pulmonary regurgitation. J Thorac Cardiovasc Surg 143:1103–1107. doi:10.1016/j.jtcvs.2011.09.039.
3. Geva T (2013) Indications for pulmonary valve replacement in repaired tetralogy of fallot: the quest continues. Circulation 128:1855–7. doi:10.1161/CIRCULATIONAHA.113.005878.
4. Galea N, Carbone I, Cannata D, et al (2013) Right ventricular cardiovascular magnetic resonance imaging: normal anatomy and spectrum of pathological findings. Insights Imaging 4:213–223. doi:10.1007/s13244-013-0222-3.
5. Babu-Narayan S V., Kilner PJ, Li W, et al (2006) Ventricular Fibrosis Suggested by Cardiovascular Magnetic Resonance in Adults With Repaired Tetralogy of Fallot and Its Relationship to Adverse Markers of Clinical Outcome. Circulation 113:405–413. doi:10.1161/CIRCULATIONAHA.105.548727.