5410
Phenotypic characterisation of multifocal cardiovascular involvement in Takayasu arteritis with cardiovascular magnetic resonance1Cape University Body Imaging Centre, University Cape Town, Cape Town, South Africa, 2Cape University Body Imaging Centre, CUBIC University Cape Town, Cape Town, South Africa, 3Division of Cardiology,Department of Medicine,University of Cape Town, University of Cape Town, Cape Town, South Africa, 4Division of Cardiology,Department of Medicine,University of Cape Town, University Cape Town, Cape Town, South Africa, 5Cape Heart Institute,Faculty of Health Sciences, University Cape Town, Cape Town, South Africa
Synopsis
Takayasu arteritis (TA) is an uncommon inflammatory disease primarily affecting the aorta and its main branches. It is more common in females (80-90% of cases) and occurs between the ages of 10 and 40 years. We report on a young male patient diagnosed with TA at age 16 years. CMR showed progressive aneurysmal dilatation of the aorta compressing the trachea, left main bronchus, and left lung. Both ventricles showed mild decrease in function (LVEF- 45% and RVEF – 51%). CMR played an important role in disease monitoring and guided patient management.
Background
Takayasu arteritis (TA) is a rare inflammatory disease of unknown aetiology, classified as a large vessel vasculitis because it primarily affects the aorta and its main branches.1,2,3,4 TA is rare in males and more prevalent in females (80-90% of cases), typically aged 10 and 40 years.1,2,3,4 Cardiovascular magnetic resonance (CMR) is useful for the evaluation of multifocal cardiac and vascular involvement in TA and has been successfully utilised to monitor disease progression.Summary
A case report in a young male with TA using magnetic resonance imaging. CMR showed advanced aortic disease with multiple small aneurysms arising from the descending thoracic aorta medial, and mild bi-ventricular dysfunction (LVEF 45% and RVEF 51%).Case report
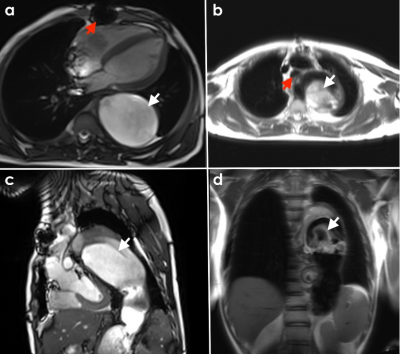
We report on a male patient with Takayasu arteritis (TA) who initially presented aged 16 years with persistent right shoulder pain and clinical findings of lower motor neuron fallout in the right arm, including muscle wasting and reduced power and reflexes. A pulsating mass at the base of his neck, on the right side, was noted, over which a bruit was auscultated. He was HIV uninfected and with a negative autoimmune screen, with inflammatory markers elevated (erythrocyte sedimentation rate (ESR) = 121, C-reactive protein (CRP) = 63). Computed tomographic (CT) angiography confirmed a dilated thoracic and abdominal aorta with multiple aneurysms of the aorta, the largest arising from the right lateral aspect of the aortic arch measuring approximately 5x5 cm, containing peripheral mural thrombus and causing compression of the trachea, right main bronchus, and the right brachial plexus. Focal stenosis was noted in the right subclavian artery followed by a segment of aneurysmal dilatation (5cm in diameter). Aneurysms were also noted in the left subclavian, external carotid, intercostal vessels, and bilateral renal arteries. The patient was referred for vascular surgery, and bilateral subclavian artery bypass grafts and thoracic aortic reconstruction (Elephant Trunk surgery) were performed. A follow-up procedure for abdominal aortic and renal artery reconstruction was planned for a later stage but the patient subsequently declined. His ambulatory care included methotrexate, prednisone, and antihypertensive therapy. Two years later, he presented with new-onset seizures and severe abdominal pain. CT brain showed evidence of multiple infarcts in keeping with thromboembolism and repeat CT angiography showed worsening enlargement of his aneurysms, and the right renal and femoral arteries were thrombosed. He subsequently agreed to surgery and was imaged with CMR. He was scanned on a 3T Siemens Magnetom Skyra (Erlangen, Germany) using a large field-of-view 18-channel body array and integrated 32-channel spine coil. The protocol used included cine imaging, T1 and T2 parametric maps, and late gadolinium enhancement (LGE).Results
CMR revealed evidence of previous median sternotomy with an anatomically normal heart and conventional systemic and pulmonic connections. The left ventricle (LV) had reduced function (LVEF 45%) with normal volumes (LVEDV 134mL, LVEDD 45mm). There was mild impairment of the right ventricular (RV) function (RVEF 51%) with normal volumes (RVEDV 108mL). T1 and T2 relaxation times were normal with no LGE present. A long segment of aortic dissection, commencing in the arch, and extending to the iliac vessels was noted, with thickening and irregularity of the aortic wall, and extensive laminated thrombus in the distal false lumen. Progressive aneurysmal dilatation of the aorta was noted from the aortic arch (ascending aorta measured 32mm, aortic arch 50mm, and proximal descending aorta 68mm) and extending to the descending thoracoabdominal aorta measuring 98mm in maximal diameter (Fig.1c). The aneurysmal dissected aorta caused significant compression of the trachea, left main bronchus, and left lung (Fig.1b). A large mural thrombus was noted within the proximal abdominal aortic aneurysm (Fig.1c). Multiple small aneurysms were noted arising from the descending thoracic aorta medial (Fig. 1d). The brachiocephalic trunk and visualized portion of the left common carotid arteries appeared normal. A bypass graft was noted originating near the origin of the right subclavian artery and inserting in the right brachial artery with the lumen appearing patent. The visualised portion of a bypass graft in the right subclavian region is patent. The right main renal artery appears thrombosed, and a small accessory vessel can be seen entering the right kidney arising from the aorta.Teaching Points
1. Magnetic resonance imaging provides excellent utility in the context of Takayasu’s arteritis with its ability to noninvasively visualise both the lumen and vessel wall with high resolution, revealing aneurysm location, shape, extension as well as vascular inflammation, stenosis, thrombus, and dissections of the aorta, its proximal branches, and other peripheral arteries.2. CMR has multiparametric strengths including the ability to assess the level of myocardial function, viability, inflammation, fibrosis, and haemodynamics.
3. CMR is an appropriate imaging modality in children and young patients with TA requiring follow-up as it is free of ionizing radiation, enabling serial evaluation as a marker of treatment response and an indicator of subsequent complications.
Conclusion
CMR is an important imaging modality in TA patients that requires repeated imaging to track disease progression over time due to its ability to provide reproducible visualization of vascular anatomy and function, as well as other multiparametric strengths.Acknowledgements
No acknowledgement found.References
1. Li Cavoli, G., Mulè, G., Vallone, M. G., & Caputo, F. (2018). Takayasu’s disease effects on the kidneys: Current perspectives. International Journal of Nephrology and Renovascular Disease, 11:225–233. Dove Medical Press Ltd.
2. Esatoglu, S. N., & Hatemi, G. (2022). Takayasu arteritis. Current opinion in rheumatology, 34(1):18–24.
3. Khalife, T., Alsac, J.M., Lambert, M., Messas, E., Duong Van Huyen, J.P., Bruneval, P., Farahmand, P., Julia, P. & Fabiani, J.N. (2011). Diagnosis and surgical treatment of a Takayasu disease on an abdominal aortic dissection. Annals of Vascular Surgery, 25(4):556.e1-556.e5.
4. Leigh, M. B., Kor, S., Czantrak, P., Sacirovic, M., Pagonas, N., Hillmeister, P., Zeidler, G., Bramlage, P., & Buschmann, I. (2018). Recanalization of bilateral axillaris/brachialis artery occlusion in a patient with Takayasu arteritis: First case report on using a novel drug-coated nitinol “chocolate” balloon catheter. Clinical Case Reports, 6(12):2490–2494.
5. Raman, S.V., Aneja, A. & Jarjour W.N. (2012). CMR in inflammatory vasculitis. Journal of Cardiovascular Magnetic Resonance.14:82.
Figures