5389
Radiomics analysis in the era of quantitative imaging1Laboratory for Biomarker Imaging Science, Graduate School of Biomedical Science and Engineering, Hokkaido University, Sapporo, Japan, 2Global Center for Biomedical Science and Engineering, Faculty of Medicine,, Hokkaido University, Sapporo, Japan
Synopsis
Keywords: Radiomics, Quantitative Imaging, Imaging analysis, feature extraction, predictive radiomics
Quantitative magnetic resonance imaging extends beyond qualitative MRI by combining both image graphics and mathematically measured intrinsic image features to determine tissue characteristics, development, pathology, etc. While MRI has practically been qualitative both in the imaging process and interpretation, quantitative image analysis involving the mapping of numerical tissue properties with nominal values obtained from healthy cohorts has proven to add extra information to MRI images.
In this educational review we discuss the rapidly evolving field of radiomics in quantitative MRI imaging. Particular emphasis is laid on development of radiomic models for MR image analysis, validation strategies and anticipated future trends.
Learning objectives
This review aims at1. Promoting quantitative MRI imaging analysis
2. Enhancing the understanding of the radiomics process in quantitative MRI analysis
3. Showcasing the potential impact of radiomics/ radiogenomics on personalized medicine
4. Highlighting the need for collaboration towards clinical implementation
5. Presenting the future prospects of radiomics in clinical implementation based on current research trajectories
Key points
· Quantitative MRI provides quantitative measurements of physical tissue parameters
· Radiomic and radiogenomic models impacts personalize medicine
· Predictive radiomics for patient care
· Need for biomarker standardization
Introduction
There is no denying the diagnostic usefulness of conventional MRI. However, several limitations exist. Conventional MRI is highly qualitative and relies on variations in contrast between areas of suspected abnormality and supposed normality to detect pathology [1]. Given that interpretation of conventional MRI is dependent on visual appraisal, there is substantial basis for subjectivity as interpretation is solely based on visual dexterity.
In quantitative MRI, numerical signal intensities extracted from qualitative images are compared with nominal values acquired from healthy cohorts. Such statistics provides basis for predicting and monitoring disease progression or remission.
What is Radiomics?
Radiomics is a rapidly evolving quantitative imaging analysis tool. It is based on the concept that medical images contain information reflective of pathology-specific processes [2] that may ordinarily be imperceptible by the visual inspection [3]. Image parameters descriptive of the pathophysiology on which radiomics is based are described as radiomic features. Examples shown in Table 1 are extractable using dedicated software towards analysis with AI methods.
The radiomic process illustrated in Figure 1 begins with image acquisition, followed by pre-processing, segmentation, feature extraction, model building and validation [4].
Potentials in MRI imaging and healthcare
The promising outlook of MRI radiomics in image analysis for diagnosis, prognosis, stratification, treatment planning and response assessment enabled by innovative AI approaches warrant expectations for clinical deployment [4,5]. In diagnosis, radiomics has successfully been applied for detecting and grading high- and low-grade glioma noninvasively [6] comparable to a fully invasive biopsy which is yet limited by sampling bias [7]. Recent studies on predictive radiomics in oncology report impressive performance of radiomics in determining prognosis and forecasting treatment outcomes prior to the onset of therapy [8,9,10]. Such predictive performance data presents key determinants for selecting treatment strategies and endpoints for personalized care. Furthermore, by combining radiomic and radiogenomic data, genomic alterations within tumor DNA can be captured in routine MRI surveillance. With data from both models, treatment strategies can be tweaked for optimal benefits [11].
Towards the era of precision medicine, patient selection for specific therapy is expected to be grounded on informed benefit to patient. Key determinants for such decisions can be supported by the clinical outlook obtained with predictive radiomics. In a similar study, Kickingereder et al [12] explored the utility of radiomics in assessing the overall survival of patients expected to be treated with bevacizumab, achieving an area under the curve (AUC) of 0.792.
In radiotherapy treatment planning, accurate segmentation of treatment volumes can be achieved by incorporating tumor and peritumoral quantitative features in radiomic analysis. Optimal doses to target volumes can be achieved while maintaining tolerable dose to peripheral healthy tissues. Although still at an elemental stage, radiomics has shown potential in quantifying hypoxia status within heterogeneous tumor post radiotherapy, [13] and this could be inclusive in-patient stratification
Areas of improvement
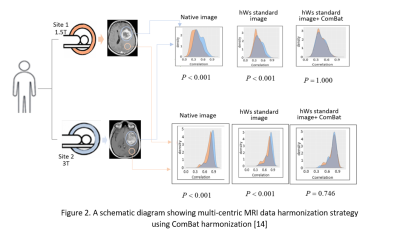
Further refinements and developments are needed ahead of clinical implementation of MRI radiomics. MRI imaging is dependent on both tissue characteristics and scanner parameters. This implies a high probability of varying results when multi-institutional data is used. A similar situation arises when same subject data is obtained from scanners with varying acquisition parameters. To optimize MRI radiomics, standardization of image acquisition protocols and harmonization of multi-centric data is necessary [14]. An approach for harmonizing multi-centric data is illustrated in Figure 2.
Future prospects
Expectations for clinical deployment is founded on well-documented model performance in research studies. Towards realizing this goal, there is a need for transferability of development around biomarker imaging science. The Quantitative Imaging Biomarkers Alliance (QIBA) whose intent is to ensure optimal performance of biomarkers in research provides two recommendations for standardization [16,17].
1. A test of repeatability: This provides guidelines for quantifying the repeatability of measured biomarkers given that all acquisitions and processing parameters are held constant. The intent is to evaluate variations given the same scanner parameters within a small-time frame.
2. Test of reproducibility: Given that acquisition or processing parameters are altered, this test evaluates the variations in measured biomarker data [18].
Based on recommendations for evaluating radiomics model performance on specialized imaging sequences, recommended acquisition parameters for diffusion-weighted and dynamic contrast enhanced MRI have been put forth by the QIBA towards optimization of imaging biomarkers derived from these sequences. These recommendations are as shown in Table 2[16].
Strategies for enhancing the repeatability and reproducibility of biomarkers towards improving radiomic analysis include intensity standardization, volume resampling, bias field correction and noise filtering [18,19].
Conclusion
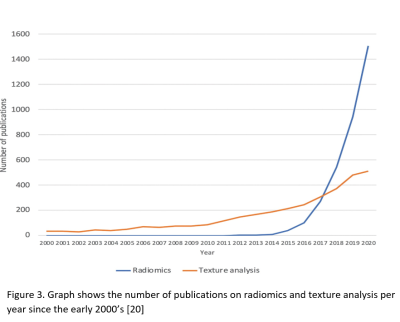
The future prospect of radiomics towards mainstream clinical utility is very encouraging. Substantial studies continue to churn out yearly. This is a representation of global interests [20].
Acknowledgements
I am grateful to all my laboratory mates and PI whose positive influence has been useful in completing this study and other related projects
References
1. Carlo P. Quantitative MRI. Top Magn Reson Imaging. 2010; 21(2):63
2. Neisius U, El-Rewaidy H, et al. Radiomic analysis of myocardial native T1 imaging discriminates between hypertensive heart disease and hypertrophic cardiomyopathy. JACC Cardiovasc Imaging. 2019; 12:1946–1954.
3. Mannil M, von Spiczak J, et al. Texture analysis and machine learning for detecting myocardial infarction in non-contrast low dose computed tomography: unveiling the invisible. Invest Radiol. 2018; 53:338–343
4. Rizzo S B, Raimondi S, et al, Radiomics: the facts and the challenges of image analysis. Eur Radiol Exp. 2018; 14:2(1):36
5. Miles K. Radiomics for personalized medicine: the long road ahead. Br J Cancer 2020; 122, 929–930
6. Kobayashi K., Miyake M., Takahashi M., Hamamoto R. Observing deep radiomics for the classification of glioma grades. Sci. Rep. 2021; 11, 10942
7. Komori T. Grading of adult diffuse gliomas according to the 2021 WHO Classification of Tumors of the Central Nervous System. Lab. Investig. 2021, 102, 126–133
8. Bortolotto C L A, et al. Radiomics features as predictive and prognostic biomarkers in NSCLC. Expert Rev Anticancer Ther. 2021; 21(3):257-266
9. Bera K., Braman N., Gupta A., et al. Predicting cancer outcomes with radiomics and artificial intelligence in radiology. Nat Rev Clin Oncol 2022; 19, 132–146
10. Bousabarah K., Blanck O., Temming S. et al. Radiomics for prediction of radiation-induced lung injury and oncologic outcome after robotic stereotactic body radiotherapy of lung cancer: results from two independent institutions. Radiat Oncol 2021; 16, 74
11. Beig N, Bera K, Tiwari P. Introduction to radiomics and radiogenomics in neuro-oncology: implications and challenges. Neurooncol Adv. 2021;2(Suppl 4): iv3-iv14.
12. Kickingereder P., Götz M., et al. Large-scale Radiomic Profiling of Recurrent Glioblastoma Identifies an Imaging Predictor for Stratifying Anti-Angiogenic Treatment Response. Clin. Cancer Res. 2016; 22, 5765–5771.
13. Loredana G. Marcu Jake C. Forster, et al. The Potential Role of Radiomics and Radiogenomics in Patient Stratification by Tumor Hypoxia Status, JACR. 2019; 16(9) B:1329-1337,
14. Hu L.S., Swanson K.R., Roadmap for the clinical integration of radiomics in neuro-oncology. Neuro-Oncology 2020; 22, 743–745.
15. Fanny O, Augustin L, et al. How can we combat multicenter variability in MR radiomics? Validation of a correction procedure. European Radiology, Springer Verlag, In press. ffhal-02945627f
16. Barnhart H X., Barboriak D P. Applications of the Repeatability of Quantitative Imaging Biomarkers: A Review of Statistical Analysis of Repeat Data Sets. Transl. Oncol. 2009; 2, 231
17. Shukla-Dave, A., Obuchowski N A, et al. Quantitative Imaging Biomarkers Alliance (QIBA) Recommendations for Improved Precision of DWI and DCE-MRI Derived Biomarkers in Multicenter Oncology Trials. J. Magn. Reson. Imaging JMRI 201; 49, e10
18. Raunig D L., McShane L M., et al. Quantitative imaging biomarkers: A review of statistical methods for technical performance assessment. Stat. Methods Med. Res. 2015; 24, 27–67
19. Carré A., Klausner G., et al. Standardization of brain MR images across machines and protocols: Bridging the gap for MRI-based radiomics. Sci. Rep. 2020; 10, 12340
20. Joshua D S, Simon J D, et al Radiomics in Oncology: A Practical Guide RadioGraphics 2021; 41:6, 1717-1732
Figures
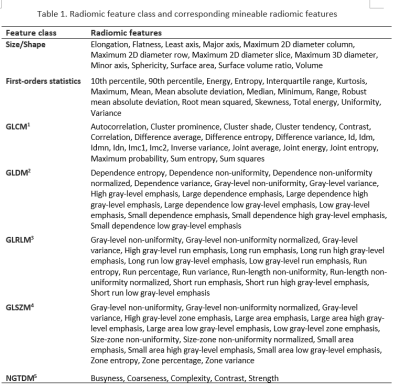
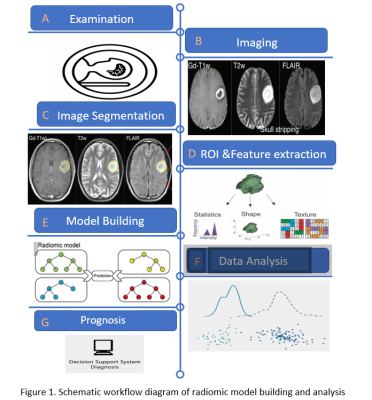
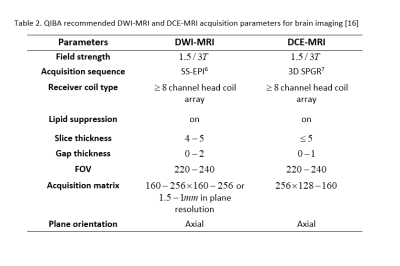
Table 2 presents QIBA acquisition parameters for optimal biomarker imaging based on specialized MRI sequences; Diffusion-Weighted Imaging (DWI) and Dynamic Contract-Enhanced (DCE) MRI imaging
From table [6] Single shot-echo planar [7] 3-Dimensional fast spoiled gradient recalled echo sequence