5371
Developing novel MRI biomarkers of amyloid beta aggregation and inflammation in APPswe/PS1dEd mouse model of Alzheimer’s Disease1Molecular Neurobiology and Neuropathology, Institute of Neuroscience of Alicante, Sant Joan d'Alacant, Alicante, Spain, 2Cellular and Systems Neurobiology, Institute of Neuroscience of Alicante, Sant Joan d'Alacant, Alicante, Spain
Synopsis
Keywords: Alzheimer's Disease, Microstructure
We present a framework to extract novel MRI biomarkers, based on diffusion-weighted contrast, capable of capturing microstructural alterations in the APPswe/PS1dE9 mouse model of Alzheimer’s disease. To validate the MRI framework, imaging was complemented with histology, with the aim of elucidating the cell-scale biological basis. We demonstrate that MRI signal carries the fingerprint of Alzheimer’s disease characteristic plaque aggregation and associated neuroinflammation patterns in a cohort of 18 months old APPswe/PS1dE9 mice as compared to healthy age-matched controls. This framework sets the basis for an in-vivo multimodal imaging protocol, translatable to humans, for early detection of Alzheimer-specific microstructural alterations.
INTRODUCTION
Alzheimer's diseaseAlzheimer’s disease is a neurodegenerative disorder characterized by progressive cognitive decline and accumulation of amyloid-plaques and neurofibrillary-tangles1. It progresses asymptomatically during decades and is belatedly diagnosed when therapeutic strategies are less viable. Since it is widely accepted that the microstructural damage begins 10-20 years before cognitive symptoms appear2, early detection has high priority. Therefore, mice models are fundamental to investigate the pathological mechanisms and test intervention. One of the most reliable models is APPswe/PS1dE9 mice containing human transgenes for both amyloid precursor protein (APP) and presenilin (PS1) genes harboring the Swedish mutation and the deletion of exon nine, which increases amyloid formation, respectively. Both genes are regulated by prion protein (Prp) promotor, driving a well-described pattern of amyloid plaque deposition that starts in the neocortex and expands towards hippocampus, beginning at 6-7 months of age3.
Inflammation progression
Amyloid-beta (Aβ) peptides form aggregates that activate microglia, which endocytose and compact them to prevent their dispersion by forming plaques. Activated microglia promote astrocyte recruitment, consequently forming a cell cluster around the plaques as a protective barrier4. Initial immune response acts thus limiting damage spread, but chronic inflammatory response leads into progressive degeneration with increasing severity as pathology evolves2.
Glial chronic activation is translated into brain-parenchyma disruption, together with vasculature permeation, synaptic dysfunction, and neuronal loss. The progression and correlation of the described microstructural changes are yet to be clarified, what highlights the need to exhaustively characterize the evolution of the different biomarkers in a specific manner for Alzheimer’s Disease.
MRI: non-invasive approach to the disease
Magnetic resonance imaging (MRI) allows characterizing microstructural integrity in vivo and non-invasively5 and is regarded as a powerful tool in the future of early detection6. Due to the early beginning of microstructural damage and inflammation, it is crucial to focus on early detection of patterns. However, conventional MRI techniques, while sensitive to microenvironment alterations, lack specificity to neurobiological substrate driving the changes. Recently, specific biomarkers to look at white7 and grey matter microstructure8 have become available. Hence, our aim was to test the capability of advanced-MRI biomarkers to capture microstructural differences between APP/PS1 mice and age-matched controls.
THEORY, ACQUISITION AND MODEL FITTING
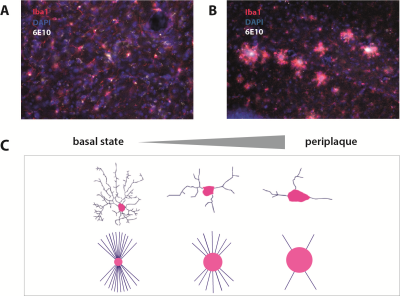
Here we develop a multimodal MRI protocol to extract conventional and advanced whole-brain imaging biomarkers. In gray matter, we measured the standard diffusion tensor MRI parameter mean diffusivity (MD) and microglia-related parameters from a more complex diffusion-weighted (dw) sequence, like microglial density FM 8 (model is illustrated In Figure 1C). In white matter, we measured fractional anisotropy (FA) and axonal density (FR).Since the biggest obstacle to MRI is the lack of specificity in transferring microstructural changes to a specific histological alteration, we sought to validate MRI parameters with post-mortem histological immunostaining for exhaustive study of microstructural changes at cellular and parenchymal level.
Acquisition
MRI experiments on mice (6 APP/PS1 and 9 controls) were performed on a 7T scanner (Bruker, BioSpect 70/30). Dw-MRI data were acquired using a Stimulated Echo sequence with 40 uniformly distributed gradient directions, b=1000 and 2500 a/mm2, diffusion times 15, 25, 40 and 60ms, three images without diffusion weight (b=0), TR=8000ms and TE=25.2ms. Matrix size= 90x 108x 16, slice thickness= 0.8 mm. A T2-weighted relaxometry sequence was acquired with the same geometry, TR=3000ms, TE=7.68ms, 4 averages.
MRI analysis and models
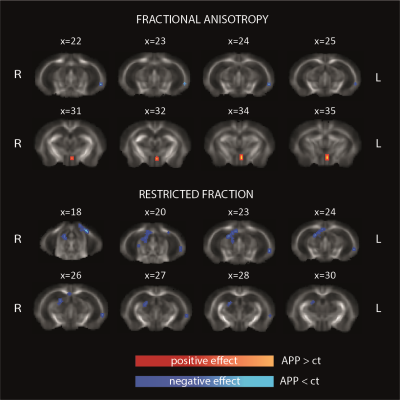
MRI data was non-linearly registered to T2W scan and corrected for distortions using a custom routine written in MATLAB (R2021a). In grey matter we measured MD and FM, in white matter we measured FA and FR. Data were processed and compared across groups in standard space using an in-house parcellation according to the Allen brain mouse atlas for grey matter, and using Tract Based Spatial Statistics in white matter. ANalysis Of VAriance (factors: region, group) was used to test for significant group and group*region effects.
Histology
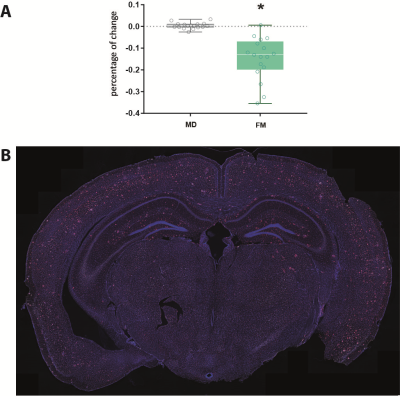
Mice were deeply anesthetized with isoflurane immediately after the scanning and intracardially perfused to extract the brain. Brains were cut in cryotome in 50 µm serial coronal sections, and stained to label microglia (Iba1), amyloid plaques (6E10) and nuclei (DAPI). Whole-brain images of a representative APP/PS1 mice were acquired using Thunder widefield microscope.
RESULTS
In cortical regions, while MD is not significantly different across groups, APP/PS1 animals have significantly reduced quiescent microglia fraction (FM) compared to controls, as shown in Figure 2A (pval=0.0119). Histology confirmed plaque formation and active periplaque microglia as shown in Figure 1A-1B, and Figure 2B.Such cortical alterations are paralleled by white matter differences in the two groups. Two white matter tract-specific imaging parameters were reported significantly altered in APP/PS1: FA, showing both reduction and increase, and FR, showing a reduction compatible with reduced axonal integrity in corpus callosum, fornix and mammillary tracts, as shown in Figure 3.
CONCLUSIONS
Our results show, in grey and white matter, increased sensitivity and specificity of advanced dw-based MRI to 1) plaque formation and glia activation in APP/PS1 mice; and 2) altered white matter microstructure, paralleling histological evidence obtained in the same animals. This framework holds great promises as a non-invasive screening to detect early microstructural alterations associated to Alzheimer’s disease-related damage.Acknowledgements
No acknowledgement found.References
1. Villain, N. & Dubois, B. Alzheimer’s Disease Including Focal Presentations. Semin Neurol 39, 213–226 (2019).
2. Sochocka, M. et al. The Gut Microbiome Alterations and Inflammation-Driven Pathogenesis of Alzheimer’s Disease—a Critical Review. Mol Neurobiol 56, 1841–1851 (2019).
3. Radde, R. et al. Abeta42-driven cerebral amyloidosis in transgenic mice reveals early and robust pathology. EMBO Rep 7, 940–6 (2006).
4. Habib, N. et al. Disease-associated astrocytes in Alzheimer’s disease and aging. Nat Neurosci 23, 701–706 (2020).
5. Yousaf, T., Dervenoulas, G. & Politis, M. Advances in MRI Methodology. in 31–76 (2018). doi:10.1016/bs.irn.2018.08.008.
6. Chandra, A., Dervenoulas, G. & Politis, M. Magnetic resonance imaging in Alzheimer’s disease and mild cognitive impairment. J Neurol 266, 1293–1302 (2019).
7. Toschi, N., Gisbert, R. A., Passamonti, L., Canals, S. & de Santis, S. Multishell diffusion imaging reveals sex-specific trajectories of early white matter degeneration in normal aging. Neurobiol Aging 86, 191–200 (2020).
8. Raquel Garcia-Hernandez, Antonio Cerdán Cerdá, Alejandro Trouve Carpena, Mark Drakesmith, Kristin Koller, Derek K. Jones, Santiago Canals, S. D. S. Mapping microglia and astrocytes activation in vivo using diffusion MRI. bioRxiv 2020.02.07.938910 doi:https://doi.org/10.1101/2020.02.07.938910.
Figures