5370
Quantitative analysis of metabolites in mouse optical track after severe traumatic brain injury using magnetic resonance spectroscopy1Georgia Cancer Center, Augusta University, Augusta, GA, United States, 2Neurosurgery, Augusta University, Augusta, GA, United States, 3Oral Biology and Diagnostic, Augusta University, Augusta, GA, United States, 4Georgia Cancer Center, Georgia medical College, Augusta, GA, United States
Synopsis
Keywords: Data Acquisition, Metabolism, Spectroscopy
Traumatic brain injury (TBI) is associated with an increased risk of cognitive and neurodegenerative complications that may develop and persist years after injury. Altered metabolism is considered to be the earliest possible sign of tissue injury and will be present before any structural changes can be detected. Magnetic resonance spectroscopy is a useful technology for longitudinal assessments, which are critical for understanding altered neurochemical concentrations following TBI, suggesting impaired neurotransmission and energy generation, neuronal injury/death, and oxidative stressIntroduction
In vivo 1H magnetic resonance spectroscopy (MRS) provides a non-invasive method to measure brain metabolites. Since abnormal metabolism is part of the pathophysiologic cascade following traumatic brain injury (TBI), MRS has been utilized to identify metabolite changes in regions of injury, to define the extent of pathology, and as a biomarker of the outcome. The extent of metabolite change differs depending on the mechanism of injury, injury severity, the region studied, and the specific acquisition technique used.
Methods
Ten mice (C57BL/6J, 10-12 weeks, 20-25g) were used in this experiment. All mice (5 male and 5 female) were anesthetized with isoflurane (2%) and subjected to a controlled cortical impact (CCI)1. Each mouse was scanned three times, baseline, 4 and 30 days following TBI. In-vivo single voxel 1H-MRS was performed on a Bruker BioSpec system (Bruker NMR, Inc., Billerica, MA) consisting of a 7-Tesla (T), 20-cm horizontal bore, superconducting magnet (Magnex Scientific, Abingdon UK) with a Biospec 70/20 console and Paravision software. An Autopac mouse positioning and physiological monitoring system, 86mm quadrature transmit coil and a 4-channel phase array mouse head coil were used. Mice were anesthetized with isoflurane during the preparation period and during the scan using a ‘flow through’ nose cone (1.5-2.0%). Single voxel 1H-MRS data were acquired using Point RESolved Spectroscopy (PRESS, TR/TE = 2500/20ms, averages = 256, voxel size = 3-5mm3), with and without water suppression, localized at the contralateral and ipsilateral optical track. Spectra were processed in LC-Model (S.W. Provencher), with eddy current correction and water-scaling, to quantify brain metabolites N-acetylaspartate (NAA), glutamate+glutamine (Glx), choline (Cho), creatine (Cr) and myo-inositol (mIns) in the selected regions. Concentrations between groups were statistically analyzed in MATLAB (The Math Works, Natick, MA) and GraphPad (GraphPad, La Jolla, CA) for significances.Results
The major results of this experiment are summarized in the tables 1 and 2. At day 4 after TBI the male group shows decrease in most of the metabolites compare to baseline control with the exception of Myo-inositol which had a minor increase of about 27% increase. The acute male TBI mice showed a large percentage decrease in NAA (62%) compare to the female groups with no significant change in the NAA (6%). At day 30, female TBI mice showed deepened loss in metabolites, while male TBI mice showed sign of improvement in terms of metabolites.Summary
This study reveals sex difference between the changes in metabolites in the brain after severe CCI in mice. Male group had reduce metabolites in acute and a small increase chronic stage compare to the baseline. The female group had maximum decrease in all the metabolite in the chronic stage with NAA and choline increasing in the acute stage. This need further investigation to look at these neural makers for stress due to the TBI for future studies and recovery after TBI.Acknowledgements
The work was funded in part by NINDS R01NS114560 and internal support from Georgia Cancer Center, Augusta UniversityReferences
1. Braun, M. et al. Activation of Myeloid TLR4 Mediates T Lymphocyte Polarization after Traumatic Brain Injury. Journal of immunology (Baltimore, Md.: 1950) 198, 3615-3626, doi:10.4049/jimmunol.1601948 (2017).
2. Andrews-Shigaki, B. et al. Quantitative analysis of metabolites in mouse brain following heat exposure using magnetic resonance spectroscopy. 2012 ISMRM annual meeting
3. Harris, J.L. et al. Probing astrocyte metabolism in vivo: proton magnetic resonance spectroscopy in the injured and aging brain. Front. Aging Neurosci. 2015, 7, 202.
Figures
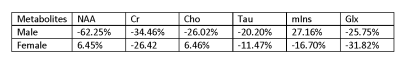
Day 4 after TBI: Average % Change of metabolites in the ipsilateral region
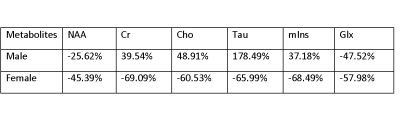
Day 30 after TBI: Average % Change of metabolites in the ipsilateral region