5363
Diffusion Time Spectrum Analysis Observation of Aquaporin Functional Dynamics1Tokyo Metropolitan University, Tokyo, Japan, 2RIKEN Center for Brain Science, Saitama, Japan, 3Keio University School of Medicine, Tokyo, Japan
Synopsis
Keywords: Diffusion/other diffusion imaging techniques, Diffusion Tensor Imaging, diffusion time
The function of water molecule exchange on the plasma membrane by aquaporins was evaluated using HEK293T cultured cells. Time-dependent diffusion magnetic resonance imaging (MRI), in which diffusion time (DT) was varied in 14 steps from 13 to 784 ms, was used to observe differences in water molecules and the permeability of the cell membrane with and without aquaporin inhibition. The diffusion coefficient increased with increasing DT in normal cells, but not in aquaporin 4-inhibited cells. These results suggest that time-dependent diffusion MRI may capture the function of water molecule exchange at the plasma membrane.Introduction
The plasma membrane contains a membrane protein called aquaporin (AQP). Although the lipid bilayer of the cell membrane is basically impermeable to water molecules, this protein allows water molecules to enter and exit the membrane.1 In vivo, the composition, concentration, and pH of inorganic ions inside and outside the cells are essentially maintained via AQPs in the cell membrane.2 Currently, 13 types of AQPs have been discovered.3 One of them is AQP4, which is a major water channel in the central nervous system and has been implicated in central nervous system (CNS) diseases such as Alzheimer’s disease.4 If the functional dynamics of AQP4 can be observed by MRI, it may lead to the development of treatments and new drugs for these diseases.5 Currently, position-emission tomography scan using [11C]TGN-020 has been proposed as a method for imaging AQP4.6 However, this method has the problem of radiation exposure. Therefore, in this study, we attempted a non-invasive approach to imaging AQP using the diffusion information of AQP. Previous studies have reported that diffusion-weighted imaging (DWI) can capture differences in cell membrane water permeability with and without AQP4 expression. However, no clear difference was captured at low b values.7 Time-dependent diffusion magnetic resonance imaging (td-dMRI) can estimate the size of structures from diffusion information by varying the diffusion time (DT) with a constant b value. This study aimed to investigate whether the intra- and extracellular water molecule exchange functions by AQPs can be captured by td-dMRI.Methods
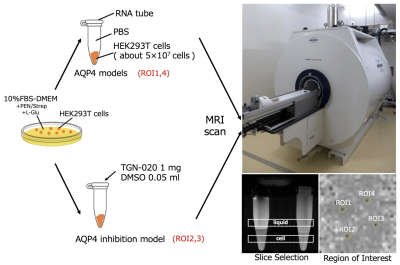
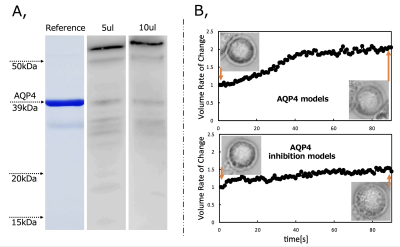
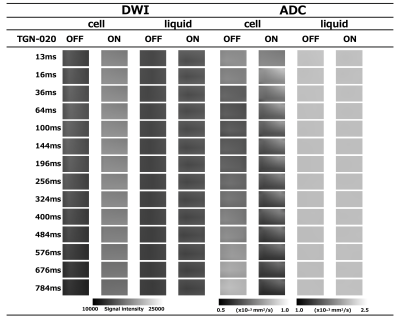
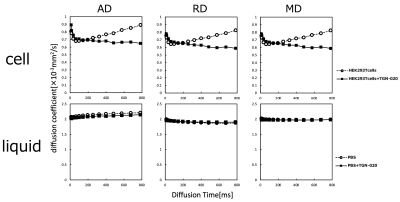
Living organisms have complex structures, and it is difficult to determine which information among the various biological structures influences MRI contrast. Therefore, we created a simple model using single cells and conducted experiments. The cells used were HEK293T cells, which are cultured cells derived from human kidney. First, Western blotting was performed to confirm the presence of AQP4 in HEK293T cells. Then, hypo-osmotic stimulation experiments were performed to confirm the efficacy of TGN-020, an AQP4 inhibitor. Cells were cultured in Petri dishes and washed with phosphate-buffered saline (PBS). Cells were then spheroidized by trypsin treatment. After this treatment, the cells were allowed to stand for 10 min until they became spherical. This allowed the volume to be calculated from the diameter of the cells. Cells were prepared with AQP4 inhibitor (AQP4 inhibitor model) and without AQP4 inhibitor (control), and the expansion of cells when 1× PBS was replaced with 0.25× PBS in a Petri dish was recorded using a microscope with a camera.In the AQP4 inhibition model, TGN-020 was dissolved in both 1× PBS and 0.25× PBS. The cell diameter was then measured using ImageJ, and the effect of the inhibitor on AQP4 kinetics was observed based on the difference in the volume change due to expansion caused by the differential water molecule exchange function through the cell membrane by AQP4. The tube containing the cells was then fixed in a holder, and MRI was performed. Cells were transferred to tubes containing PBS and allowed to precipitate; TGN-020 was dripped into the AQP4 inhibitor model, but not into the control. In this experiment, a STE-DWI sequence was used, and multi-axis DWI was conducted by changing the (T) in 16 steps from 13 to 784 ms (13, 16, 36, 64, 100, 144, 196, 256, 324, 400, 484, 576, 676, and 784 ms). Diffusion tensor analysis was performed using the diffusion toolkit, and radial diffusivity (RD) was calculated for each DT. The region of interest was set in each tube using ImageJ, and RD values were measured. A 9.4-T MRI (Bruker) and a solenoid coil (Takashima Seisakusho) were used for the measurements. This study was approved by the Research and Experimental Committee of RIKEN, the research implementing organization.Results and Discussion
Western blotting confirmed the presence of AQP4 in cells. In hypo-osmotic stimulation experiments, AQP4 inhibitor cells showed smaller volume expansion than normal cells. These results confirm the drug efficacy of TGN-020, an AQP4 inhibitor. PBS was mixed with TGN-020 dissolved in DMSO with boiling water. However, the increase in osmolality caused by the mixing of these in PBS was slight and considered negligible. Td-dMRI showed that the diffusion coefficient increased with increasing DT in normal cells, but not in cells in which AQP4 was inhibited. No difference in diffusion coefficient was observed in the supernatant PBS slices (liquid) with or without TGN and with or without DT extension. This may be because the diffusion of water molecules across the plasma membrane occurs in normal cells with a functioning AQP4, and when AQP4 is inhibited, the diffusion of water molecules across the plasma membrane is restricted. These results suggested that AQP4-mediated intracellular and extracellular water exchanges may be captured by td-dMRI.Acknowledgements
This work was supported by the program for Brain Mapping by Integrated Neurotechnologies for Disease Studies (Brain/MINDS) from the Japan Agency for Medical Research and Development (AMED), JSPS KAKENHI, and by “MRI platform” as a program of Project for Promoting public Utilization of Advanced Research Infrastructure of the Ministry of Education, Culture, Sports, Science and Technology (MEXT).References
[1] Takata Kuniaki, Toshiyuki Matsuzaki, Yuki Tajika. Aquaporins: water channel proteins of the cell membrane. Progress in histochemistry and cytochemistry 39.1 (2004): 1-83.
[2] Verkman, A. S. Aquaporins in clinical medicine. Annual review of medicine 63 (2012): 303.
[3] Ikarashi Nobutomo, Risako Kon, Kiyoshi Sugiyama. Aquaporins in the colon as a new therapeutic target in diarrhea and constipation. International Journal of Molecular Sciences 17.7 (2016): 1172
[4]Inês Silva, Jéssica Silva, Rita Ferreira et al. Glymphatic system, AQP4, and their implications in Alzheimer’s disease. Neurological Research and Practice 3.1 (2021): 1-9.
[5] Yang, Canhong, Huang, Xiaomin, Huang, Xiaoyu et al. Aquaporin-4 and Alzheimer’s disease. Journal of Alzheimer's Disease 52.2 (2016): 391-402.
[6]Yukihiro Nakamura, Yuji Suzuki, Mika Tsujita et al. Development of a novel ligand, [11C] TGN-020, for aquaporin 4 positron emission tomography imaging. ACS Chem Neurosci. 2011 Oct 19;2(10):568-571.
[7]Imaizumi, Akiko, Takayuki Obata, Jeff Kershaw et al. Quantitative measurement of diffusion-weighted imaging signal using expression-controlled aquaporin-4 cells: Comparative study of 2-compartment and diffusion kurtosis imaging models. PloS one 17.4 (2022): e0266465.
Figures