5360
MRI-Driven Lorentz Force-based Mechanical Removal of Arterial Occlusions1Physical Intelligence, Max Planck Institute for Intelligent Systems, Stuttgart, Germany, 2Mechanical Engineering, Carnegie Mellon University, Pittsburgh, PA, United States, 3Institute for Biomedical Engineering, ETH Zurich, Zurich, Switzerland, 4College of Engineering and School of Medicine, Koç University, Istanbul, Turkey
Synopsis
Keywords: Atherosclerosis, Atherosclerosis, Mechanical Thrombectomy
MR angiography provides high resolution visualization of blood vessels for analyzing arterial occlusions. However, these conditions require the use of a guidewire/catheter for treatment. Catheters integrated with microcoils for actuation under the high (3-7 Tesla), uniform magnetic field within magnetic resonance (MR) scanners have enabled mechanical removal of arterial occlusions. This work introduces an electromagnetic rotablation design, allowing direct tip torque control for both steering and drilling. Results demonstrate in vitro rotational thrombectomy with torque outputs up to 12 mN·m under MR guidance. These results indicate the high MRI external field can provide rotablation for difficult-to-reach areas of the vasculature.Introduction
Cardiovascular disease (CVD) is the leading cause of death worldwide according to the World Health Organization. One of the most common complications in CVDs known as Deep Vein Thrombosis (DVT), is highly dangerous because clot formation can break off and travel to the lung causing a pulmonary embolism (PE). For high-risk PE in which thrombolysis is too dangerous, mechanical thrombectomy is usually recommended. Mechanical thrombectomy includes fragmentation in which a mechanical device is used to break up thrombi in pulmonary arteries. Arterial stenosis is also a dangerous disease caused by calcified deposits that form within the arteries to restrict flow. Commercial systems exist such as the TurboHawk (Medtronic) or Diamondback Atherectomy system (Cardiovascular Systems) but are limited by their high-frequency drilling speeds [2]. In this work, we present the first MRI-driven, Lorentz-force based direct current electric motor manufactured out of MRI-compatible components for mechanical removal of arterial occlusions. The motor operates similarly to a direct current brush motor, however, the stator (which is typically a permanent magnetic housing), has been replaced by the B0 field of the MRI. This allows for direct torque transmission on the catheter tip without influencing the rest of the soft body.Methods
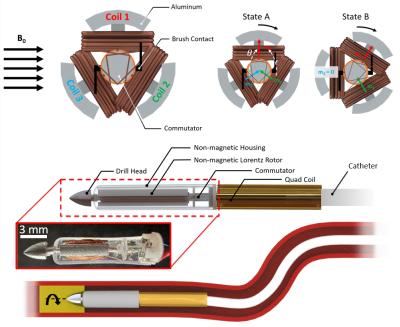
The catheter driller was manufactured using aluminum for both the drill bit and rotor while the housing and commutator were 3D printed. All components were then assembled together using cyanoacrylate and then heat shrunk to the catheter tip. Catheter driller motion was simulated according to the following equations:$$τ_{out} = m \times B_o$$
$$τ_{out} = m_1 B_0 cosωt β+m_2 B_0 sin(ωt+2π/3)β+m_3 B_0 sin(ωt+2π/3)β$$
$$β = sin α+cosα$$
$$m_1 = m_2 = m_3 = NIAB_0$$
$$τ_{out} = NIAB_0 (cosωt+2 sin(ωt+ 2π/3)β)$$
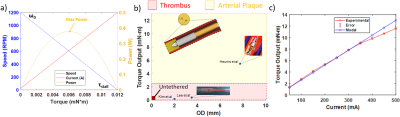
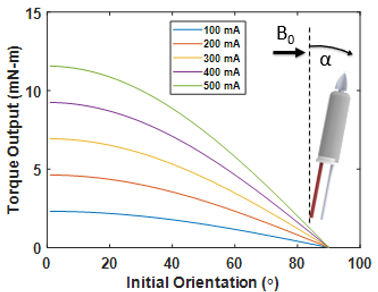
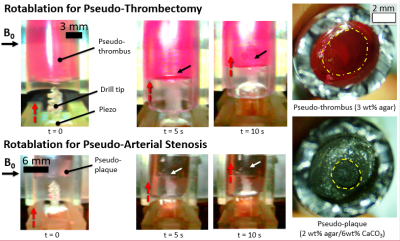
where N is the number of coil loops, I is the coil current, A represents the area of a single coil loop, and B0 represents the magnetic field vector of the MRI scanner. To validate the model equations, the MRI-driven electric motor performance was characterized at the maximum performance 90° with respect to the B0 field. Currents up to 500 mA were applied with a 3D printed lever arm mounted across onto a nonmagnetic force sensor (Futek) to measure torque output. Motor rotation performance was measured with a high-speed camera under the 7 Tesla preclinical scanner (Bruker 70/30 BioSpec). To measure the drilling performance of the catheter motor, the driller was mounted to a 3D printed fixture with a linear piezoelectric actuator (Piezomotor) to drive motion. Silicone tubes of 6 mm in diameter were used to create pseudo-thrombi using a mixing ratio of 3 wt % agar mixed with red dye, and repeated again using 2 wt % /6 wt % CaCO3 to replicate arterial plaque with similar currents as stated previously.
Results
Results from drilling characterization demonstrated a maximum torque output of 12 mN·m. A standard DC motor performance curve was constructed using the maximum rotational rate of the motor with no load speed and maximum torque output. Figure 2 demonstrates a maximum rotational rate of 1200 RPM and maximum torque output of 12 mN·m. These results closely aligned with the numerical model, demonstrating a percent error of 8% across all trials. The maximum power output is shown to be 0.5 W at half torque-speed output. Given the dimensions of the proposed driller, the torque output remains to be above literature standards (5.5 mN·m) at worst-case scenarios (angles closely aligned with the B0 field vector) as shown in Figure 3. Figure 4 demonstrates the effectiveness of drilling through pseudo-thrombus and plaque. Occlusion removal was visually observed for both samples after 10 seconds of drilling.Discussion
This work has demonstrated the feasibility of making an MRI-driven DC electric motor to be integrated to the distal end of a catheter for both steering into confined workspaces and removing arterial occlusions under MR guidance. The final prototype was machined out of nonmagnetic components (aluminum) to reduce the chances of artifacts or unintended forces/torques during steering. The maximum torque output was sufficient to drill through thrombus and plaque within reasonable time frames compared to literature. The proposed numerical model has shown to be accurate within 8 % error, which enables further optimization for down-sizing. In vitro experiments replicated thrombus and plaque densities but future work could be done using real porcine blood to also evaluate heating. However, current inputs in this work remained within FDA guidelines of 1.2 W. Future experiments should also include MR guided drilling with guidewire integration instead of direct piezo actuation as well as fully-submerged drilling under simulated flow to explore possible clotting effects.Conclusion
Utilization of the strong, permanent, magnetic field within MRI scanners has proven to be an effective method for direct catheter tip torque control. We have designed and developed the strongest existing catheter driller in the literature for both steering and drilling. The device has been designed out of MRI-compatible (non-ferrous) materials to fit within the cardiovascular system (~ 3 mm diameter) for occlusion removal. In vitro experiments demonstrated thrombus and plaque removal within 10 seconds along with torque outputs twice that of existing catheter drillers in literature. Continued development on MRI-driven rotational catheter drilling will enable unique opportunities for cardiovascular MR imaging.Acknowledgements
This work was funded by the Max Planck Society.References
1. Rousseau, Hervé, et al. "Endovascular therapies for pulmonary embolism." Heliyon 7.4 (2021): e06574.
2. Heunis, Christoff M., et al. "Design and evaluation of a magnetic rotablation catheter for arterial stenosis." IEEE/ASME transactions on mechatronics 27.3 (2021): 1761-1772.
3. Yaeger, Kurt, et al. "A technical comparison of thrombectomy vacuum aspiration systems." Journal of NeuroInterventional Surgery 12.1 (2020): 72-76.
4. Cowled P, Fitridge R. Pathophysiology of Reperfusion Injury. In: Fitridge R, Thompson M, editors. Mechanisms of Vascular Disease: A Reference Book for Vascular Specialists [Internet]. Adelaide (AU): University of Adelaide Press; 2011. 18. Available from: https://www.ncbi.nlm.nih.gov/books/NBK534267/
Figures