5351
Predictive models for early recurrence of hepatocellular carcinoma without microvascular invasion in patients after hepatectomy1Affiliated Nantong Hospital 3 of Nantong University, Nantong Third People’s Hospital, Nantong, Jiangsu, China, Nantong, China, 2Philips Healthcare, Shanghai, China, Shanghai, China
Synopsis
Keywords: Liver, Cancer
To assess the predictive value of preoperative gadoxetic acid (GA)-enhanced magnetic resonance imaging (MRI) features and postoperative histopathological grading for early recurrence of hepatocellular carcinoma (HCC) without microvascular invasion (MVI) after curative hepatectomy. The results of the present demonstrated that our predictive model incorporating postoperative Edmondson-Steiner grade and preoperative imaging features including peritumoral hypointensity on HBP and RIR on HBP (Model-2) represents a promising model to assess the risk of early recurrence after resection of MVI-negative HCC. This predictive model may help clinicians formulate more aggressive and personalised treatment plans way earlier to improve patient prognosis and reduce early recurrence.INTRODUCTION
MVI-negative HCC cases, an important subset of patients who may undergo curative liver resection, are also susceptible to early recurrence. However, studies that specifically evaluate predictors of poor postoperative prognoses (e.g., early recurrence) in MVI-negative HCC remains limited.1,2 In addition to the postoperative histopathological grading,1 preoperative MRI features2 have been reported to be correlated to early relapse and poor survival in HCC patients without MVI; these imaging features include as mosaic architecture, larger tumor size, and non-smooth tumor margins. Of note, imaging features of the hepatobiliary phase (HBP) was not incorporated in that study due to limitations of the contrast agent, gadolinium-diethylenetriamine pentaacetic acid (Gd-DTPA).2 Therefore, the parameters and predictive models involving imaging and histopathological data remain to be perfected and validated so that reliable predictive models can be made available to clinicians for them to better implement adjuvant therapy and to design clinical trials. To that end, the present retrospective study was aimed to establish a refined predictive model to predict early recurrence in MVI-negative HCC patients, by comparing a preoperative prediction model solely based on gadoxetic acid (GA)-enhanced MRI parameters (Model-1) and an integrated model involving both preoperative MRI features and postoperative histopathological measures (Model-2).METHODS
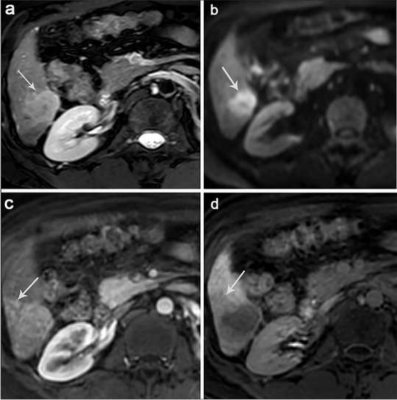
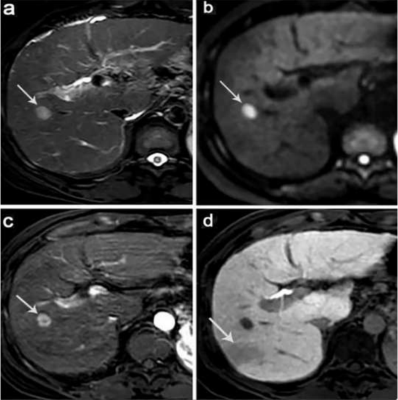
A total of 85 MVI-negative HCC cases were retrospectively analyzed, among which 28 showed early recurrence of HCC within 24 months after surgery and 57 did not exhibit recurrent HCC during the cut-off latency (24 months). All patients were performed non-enhanced and Gd-EOB-DTPA-enhanced MRI of liver. Abdominal MRI was performed using a Philips 3.0 T Achieva MR scanner with a 16-channel abdominal coil. In Gd⁃EOB⁃DTPA⁃enhanced MRI, qualitative indicators including whether the tumor signal was uniform, peritumoral enhancement, tumor capsule, tumor margin, peritumor hypointensity and presence of fat in the tumor were assessed, etc. Quantitative indicators including the relative intensity ratio (RIR) of post⁃enhancement arterial phase, portal vein phase, transitional phase and hepatobiliary phase were assessed and recorded. Interreader agreement was evaluated by using intraclass correlation coefficient (ICC).RESULTS
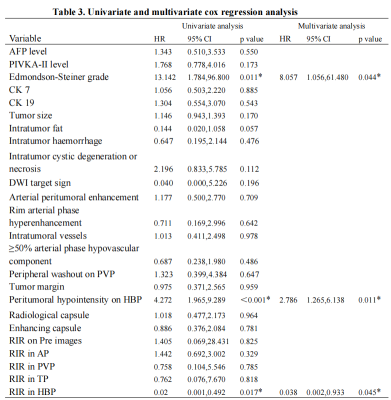
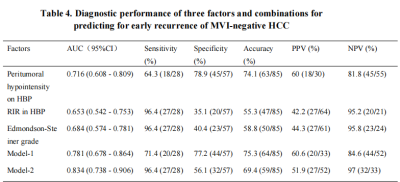
Edmondson-Steiner grade, peritumoral hypointensity on hepatobiliary phase (HBP), and RIR in HBP were identified as independent variables associated with early recurrence. The C-index of the nomogram models and internal validation were both between 0.7 and 0.8, showing good model fitting and calibration effects. The area under the ROC curve (AUC) was 0.781 for Model-1 based on the two preoperative MRI factors. When a third factor, the Edmondson-Steiner grade, was included (Model-2), the AUC increased to 0.834, and the sensitivity increased from 71.4% to 96.4%.DISCUSSION
we found that HBP peritumoral hypointensity was a useful predictor for the early recurrence of MVI-negative HCC, and this sign could be observed more frequently in the ER group. In view of the correlation between GA-enhanced MRI, an indirect molecular imaging method, and the molecular biology of tumor regulatory mechanisms,3 we believed that this sign may reveal the malignant potential of tumors. However, in this study, the sensitivity of peritumoral hypointensity on HBP to predict early recurrence of MVI-negative HCC was relatively low. We speculated that this may be because the tumor was not accompanied by microvascular invasion and that changes in blood perfusion and hepatocyte function around the tumor were relatively small. Studies have reported that poorly differentiated tumors have a negative impact on the recurrence risk and long-term survival of patients with HCC after radical liver resection.4,5 Under the Edmondson-Steiner grading system, MVI-negative HCC with different degrees of differentiation have been confirmed to be significantly associated with poor postoperative outcomes.1 This is consistent with our study where patients with poorly differentiated HCC appeared more prone to early recurrence than those with well-differentiated/moderately differentiated HCC. Studies have shown that HCC tissues with low expression of OATP1B3 potentially have an advanced TNM stage, a lower degree of tumor differentiation, and a higher risk of recurrence.6,7 Based on this conclusion, which attracted us to further explore this biomarker, new ideas for individualised medicine in liver cancer treatment may germinate. In the multivariate analysis, the quantitative imaging parameter RIR value in the hepatobiliary phase was negatively correlated with the postoperative ER; that is, with a decrease in the RIR value, the ER rate increased significantly. Ye et al8 showed that HCC with a lower tumor-to-liver SI ratio on HBP exhibited significantly reduced OATP expression levels and worse prognosis, including higher aggressiveness, higher tumor grade, and shorter recurrence-free survival. We postulated that this quantitative index could establish a correlation between the imaging features and the biological behaviour of tumors. It may be possible to non-invasively and effectively predict tumor response to treatment and the likelihood of early recurrence. The emergence of some quantitative parameters may represent greater value in predicting early postoperative recurrence of HCC, which deserved future investigation.CONCLUSIONS
Edmondson-Steiner grade, peritumoral hypointensity on HBP, and RIR in HBP can help predict early recurrence of MVI-negative HCC. In comparison to Model-1 (only imaging features), Model-2 (imaging features+ histopathological grades) increases the sensitivity in predicting early recurrence of HCC without MVI.Acknowledgements
Thanks to all the people who have devoted their time and efforts to this article, especially Professor Zhang Xueqin and Professor Zhang Tao, and thanks to Philips for providing technical support for this research.References
1. Zhou L, Rui JA, Zhou WX, et al. Edmondson-Steiner grade: A crucial predictor of recurrence and survival in hepatocellular carcinoma without microvascular invasio[J]. Pathol Res Pract, 2017, 213:824-30.
2. Wei Y, Pei W, Qin Y, et al. Preoperative MR imaging for predicting early recurrence of solitary hepatocellular carcinoma without microvascular invasion[J]. Eur J Radiol, 2021, 138:109663.
3. Kitao A, Matsui O, Yoneda, et al. Gadoxetic acid-enhanced MR imaging for hepatocellular carcinoma: molecular and genetic background[J]. Eur Radiol, 2020, 30:3438-47.
4. Shen J, Liu J, Li C, et al. The Impact of Tumor Differentiation on the Prognosis of HBV-Associated Solitary Hepatocellular Carcinoma Following Hepatectomy: A Propensity Score Matching Analysis[J]. Dig Dis Sci, 2018, 63:1962-69.
5. Chen W, Zhang Z, Fang X, et al. Prognostic value of the ALBI grade among patients with single hepatocellular carcinoma without macrovascular invasion[J]. Medicine (Baltimore), 2021, 100:e26265.
6. Kitao A, Matsui O, Yoneda N, et al. The uptake transporter OATP8 expression decreases during multistep hepatocarcinogenesis: correlation with gadoxetic acid enhanced MR imaging[J]. Eur Radiol, 2011, 21:2056-66.
7. Chen S, Li K, Jiang J, Wang X, Chai Y, Zhang C, et al. Low expression of organic anion-transporting polypeptide 1B3 predicts a poor prognosis in hepatocellular carcinoma[J]. World J Surg Oncol, 2020, 18:127.
8. Ye Z, Cao L, Wei Y, et al. Preoperative prediction of hepatocellular carcinoma with highly aggressive characteristics using quantitative parameters derived from hepatobiliary phase MR images[J]. Ann Transl Med, 2020, 8:85.
Figures