5350
Mapping astrogliosis in the individual human brain using multidimensional MRI1National Institute on Aging, Baltimore, MD, United States, 2Uniformed Services University of the Health Sciences, Bethesda, MD, United States, 3National Institute of Child Health and Human Development, Bethesda, MD, United States
Synopsis
Keywords: Traumatic brain injury, Microstructure
There are currently no noninvasive imaging methods available for astrogliosis mapping in the brain despite its essential role in the response to many disease states. In an ex vivo human brain study we used diffusion-relaxation MRI to derive a signature of astrogliosis and disentangle it from normative brain at the individual level using machine learning. We developed a within-subject anomaly detection procedure that generates MRI-based astrogliosis maps ex vivo, which were significantly and strongly correlated with co-registered histology. Our findings demonstrated spatial sensitivity and specificity in detecting reactive astrocytes, and could significantly impact the studying of injury, disease, and aging.Introduction
Astrogliosis plays an essential role in the response to many disease states, such as infarcts, neurodegenerative conditions, traumatic brain injury, and infection. Despite that, the successful development of MRI methods to image astrogliosis has been elusive, mainly because of insensitivity, but also due to the experimental difficulty of disentangling astrogliosis from co-morbid pathologies. The latter is especially true in MRI and DTI studies involving traumatic brain injury animal models that result in axonal injury, demyelination, neurodegeneration, edema, or neuroinflammatory processes that are concurrent with astrogliosis.1-5 Multidimensional MRI is an increasingly employed imaging modality that maximizes the amount of encoded chemical and microstructural information by probing relaxation (T1 and T2) and diffusion mechanisms simultaneously.6,7 Here, we harness the sensitivity of this imagining modality to derive a signature of astrogliosis and disentangle it from normative human brain at the individual level using machine learning.Methods
We investigated fixed ex vivo cerebral cortical tissue specimens derived from seven subjects who sustained blast induced injuries, which resulted in scar-border forming astrogliosis without being accompanied by other types of neuropathologic abnormality, and from seven control brain donors. Multidimensional MR data spanned by T1 and T2 (i.e., T1-T2), by T1 and mean diffusivity (i.e., T1-MD), and by T2 and mean diffusivity (i.e., T2-MD), with 56, 302, and 302 images, respectively, were acquired at 200x200x300µm3 resolution on an 7T Bruker MRI scanner according to a previously published sampling scheme.8 Multidimensional MRI data were denoised9 and processed as previously described.8,10 Following acquisition of MRI data, tissue specimens were sectioned serially into sections and stained for glial fibrillary acidic protein (GFAP) to evaluate presence of astrogliosis, for amyloid precursor protein (APP) for the detection of axonal injury, for abnormally phosphorylated tau (AT8) protein, and myelin basic protein (MBP) to evaluate possible myelin loss. Two sections per antibody were stained at 300µm apart from each other, in accordance with the MRI slice thickness. MRI-histology co-registration was performed using previously published methods.8Results
Scar-border forming astrogliosis pathology is demonstrated in immunostained sections for GFAP from four representative cases in Fig. 1. The astrogliosis pathology in our cohort was notably present at the gray-white matter junction in WM, without associated accumulation of phosphorylated Tau in involved cortical regions and did not coexist with axonal injury or with demyelination that would be indicated by APP and MBP immunohistochemistry, respectively.Multidimensional MRI data reveal that a distinct signature exists for scar-border forming astrogliosis, which cannot be seen using one-dimensional MRI measurements. Summarized data of the T2-MD contrast from representative control and injured subjects are shown in Figs. 2 A and B, respectively. In addition, the marginal distributions of subvoxel MD values (top row) and subvoxel T2 values (right column) are shown to illustrate the information content of any 1D approach. The GFAP histological image of each case is also shown on the upper-left corner of each panel, for reference. A clear separation of gray (blue frame) and white matter (green frame) can be seen in both control and injury states. However, we identified a distinct diagonal T2-MD spectral region (pink frame, Fig. 2D) in which intensities are concentrated at the gray-white matter junction, primarily on the WM side; these intensities follow closely the GFAP histological pattern (see inset image in Fig. 2B), while this newly found spectral information is absent in the control subject (Fig. 2C). Furthermore, the diagonal pattern in T2-MD points directly at a joint dependency with respect to T2 and MD, making it clear that this unique injury-related information cannot be seen by looking at T2 or MD separately. Averaged normal-appearing WM, GM, and astrogliosis T2-MD spectra across the entire study are shown in Fig. 3E left to right, respectively.
We developed a machine learning strategy to detect anomaly in individuals and consequently map astrogliosis (Fig. 3), which was used separately on each subject and with each of the T2-MD, T1-MD, and T1-T2 datasets. Figure 4 shows these multidimensional MRI maps, along with conventional MRI and DTI maps, and histological GFAP density images of six representative control and injured cases. Further, we performed radiological–pathological correlation analyses with histological GFAP density and all the investigated MRI parameters (Fig. 5).
Discussion
This is the first report of an MRI framework to directly map astrogliosis in individual brains ex vivo. In this study we showed that astrogliosis induces microstructural and compositional changes that result in a distinct multidimensional MRI spectral signature. Further, we developed a novel approach to utilize this information and obtain MRI maps of astroglial neuropathology in individual human brains. We found that the multidimensional MRI astrogliosis biomarker maps are significantly and strongly correlated with co-registered histological images of increased GFAP expression. We showed that our approach has the spatial sensitivity to detect altered tissue states at the individual level by establishing the distinction of interface astrogliosis spectral signature from normal gray-white matter interface, and by comparing normal-appearing and histologically confirmed regions within the same brain. This work emphasizes the importance and the potential of combining relaxation and diffusion MRI with artificial intelligence for studying human brain astroglial reactivity noninvasively.Acknowledgements
This research was partially supported by a grant from the U.S. Department of Defense, Program Project 308430 USUHS. Support for this work also included funding from the U.S. Department of Defense to the Brain Tissue Repository and Neuropathology Core, Center for Neuroscience and Regenerative Medicine (CNRM). DB was supported by the CNRM Neuroradiology-Neuropathology Correlation Core. DP, DPP, and DLB were supported by the CNRM and USUHS. This research was supported in part by the Intramural research Program of the NIH, National institute on Aging, and the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
References
1. Schwartz, E. D., Duda, J., Shumsky, J. S., Cooper, E. T., & Gee, J. (2005). Spinal Cord Diffusion Tensor Imaging and Fiber Tracking Can Identify White Matter Tract Disruption and Glial Scar Orientation Following Lateral Funiculotomy. Journal of Neurotrauma, 22(12), 1388–1398.
2. Budde, M. D., Janes, L., Gold, E., Turtzo, L. C., & Frank, J. A. (2011). The contribution of gliosis to diffusion tensor anisotropy and tractography following traumatic brain injury: validation in the rat using Fourier analysis of stained tissue sections. Brain, 134(8), 2248–2260.
3. Zhuo, J., Xu, S., Proctor, J. L., Mullins, R. J., Simon, J. Z., Fiskum, G., & Gullapalli, R. P. (2012). Diffusion kurtosis as an in vivo imaging marker for reactive astrogliosis in traumatic brain injury. NeuroImage, 59(1), 467–477.
4. Chary, K., Nissi, M. J., Nykänen, O., Manninen, E., Rey, R. I., Shmueli, K., Sierra, A., & Gröhn, O. (2021). Quantitative susceptibility mapping of the rat brain after traumatic brain injury. NMR in Biomedicine, 34(2).
5. Benjamini, D., Hutchinson, E. B., Komlosh, M. E., Comrie, C. J., Schwerin, S. C., Zhang, G., Pierpaoli, C., & Basser, P. J. (2020). Direct and specific assessment of axonal injury and spinal cord microenvironments using diffusion correlation imaging. NeuroImage, 221, 117195.
6. Benjamini, D., & Basser, P. J. (2020). Multidimensional correlation MRI. NMR in Biomedicine, 33(12).
7. Slator, P. J., Palombo, M., Miller, K. L., Westin, C., Laun, F., Kim, D., Haldar, J. P., Benjamini, D., Lemberskiy, G., de Almeida Martins, J. P., & Hutter, J. (2021). Combined diffusion‐relaxometry microstructure imaging: Current status and future prospects. Magnetic Resonance in Medicine.
8. Benjamini, D., Iacono, D., Komlosh, M. E., Perl, D. P., Brody, D. L., & Basser, P. J. (2021). Diffuse axonal injury has a characteristic multidimensional MRI signature in the human brain. Brain, 144(3), 800–816.
9. Benjamini, D., Bouhrara, M., Komlosh, M. E., Iacono, D., Perl, D. P., Brody, D. L., & Basser, P. J. (2021). Multidimensional MRI for Characterization of Subtle Axonal Injury Accelerated Using an Adaptive Nonlocal Multispectral Filter. Frontiers in Physics, 9.
10. Benjamini, D., & Basser, P. (2016). Use of marginal distributions constrained optimization (MADCO) for accelerated 2D MRI relaxometry and diffusometry. Journal of Magnetic Resonance, 271, 40–45.
Figures
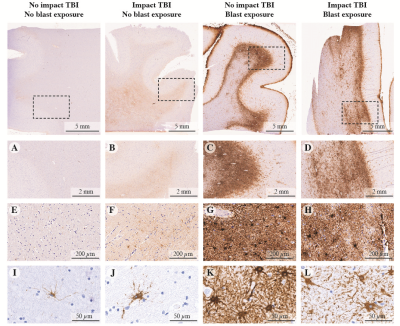
GFAP immunoreactivity in specimens without impact or blast exposure TBI, with impact TBI but without blast exposure, without impact TBI but with blast exposure, and with both impact and blast exposure TBI cases, at different magnification levels (x2, x20, and x80, from top to bottom). From left to right: minimal GFAP immunoreactivity; limited GFAP immunoreactivity with mild reactive astrocytes; dense scar-border forming astrogliosis at the grey–white matter junction; dense scar-border forming astrogliosis at the grey–white matter junction.
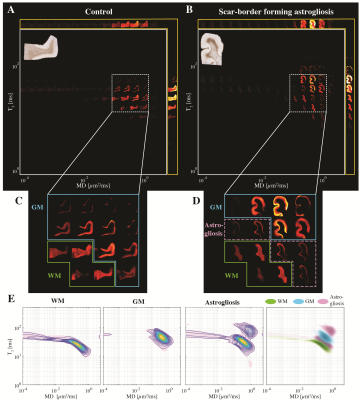
Changes in the T2-MD multidimensional MR signature induced by confirmed astrogliosis. Maps of 2D spectra of subvoxel T2-MD values reconstructed on a 16x16 grid of a representative (A) control and (B) injured subjects, along with their respective GFAP image. (C)-(D) Clear separation of white (yellow frame) and gray (teal frame) matter, with distinct spectral components at the gray-white matter interface (purple frame). (E) T2-MD spectra averaged across all subjects in WM, GM, and GFAP-positive ROIs, and a superposition of the average spectra from the three ROIs.
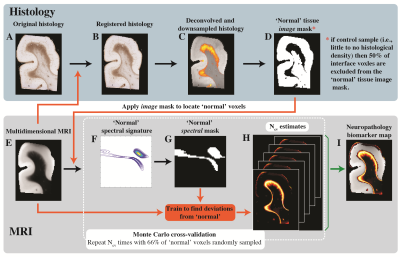
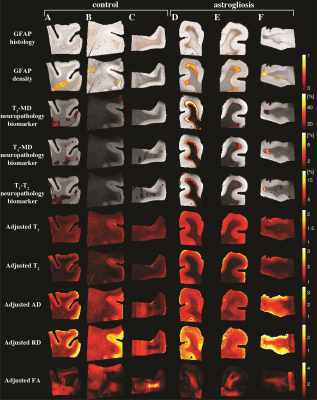
Multidimensional and voxel-averaged MRI maps. (A)-(C) are subjects without severe astrogliosis, while (D)-(F) had substantial GFAP over-expression. Different MRI contrasts, including all the conventional relaxation and DTI parameters, and the proposed multidimensional astrogliosis maps are shown, along with co-registered histological GFAP images and density maps. Multidimensional neuropathology maps overlaid onto proton density images show substantial injury along the gray-white matter interface, while conventional MRI maps do not show visible abnormalities.
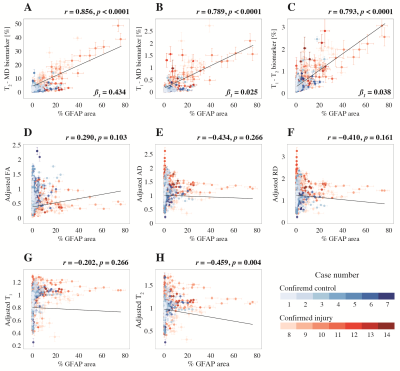
Radiological-pathological correlations between MRI metrics and GFAP density. GFAP density (% area) from 556 tissue regions from 14 subjects (color-coded, see legend) and the corresponding MR parameter correlations. Individual data points represent the mean value from each postmortem tissue sample. Scatterplots of the mean % area GFAP and (A) T2-MD, (B) T1-MD, and (C) T1-T2 injury MRI biomarkers show strong positive and significant correlation with GFAP density. The conventional MRI metrics in (D)-(H) did not result in strong and significant correlations with % area GFAP.