5349
Blood-brain barrier damage and new-onset refractory status epilepticus: an explorative study using dynamic contrast-enhanced MRI1Department of Neurocritical Care, The Second Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou, China, 2Department of Imaging, The Second Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou, China, 3Hubei University of Chinese Medicine, Wuhan, China, 4Philips Healthcare, Guangzhou, China
Synopsis
Keywords: Epilepsy, DSC & DCE Perfusion, new-onset refractory status epilepticus; status epilepticus
This is the first study investigating blood-brain barrier (BBB) dysfunction in new-onset refractory status epilepticus (NORSE) using dynamic contrast-enhanced MRI (DCE-MRI). BBBs of NORSE patients were impaired diffusely, and they had significantly higher BBB permeability in the basal ganglia and thalamus compared to encephalitis patients without status epilepticus (SE). Our preliminary findings demonstrate that BBB dysfunction in the basal ganglia and thalamus plays an important role in the pathophysiology of NORSE.INTRODUCTION
Status epilepticus (SE) has a high mortality rate of up to 47%1, 2, yet unfortunately, 31% to 45% of SE cases cannot be controlled by adequate doses of an initial benzodiazepine and second-line antiepileptic drugs3, 4, which is defined as refractory SE (RSE)5. Accounting for 19% of RSE cases6, new-onset RSE (NORSE) occurs in previously healthy individuals and usually has a negative initial workup, presenting a challenge when implementing a targeted treatment. Despite an extensive workup, 52% to 73% of NORSE cases remain cryptogenic6, 7. Dynamic contrast-enhanced MRI (DCE-MRI) is a burgeoning imaging technique that measures the integrity and leakiness of tissue vasculature and has been used in neuroscience to quantify the disruption of the blood-brain barrier (BBB)8-10. Two clinical studies assessed the integrity of BBB in SE patients by measuring cerebrospinal fluid (CSF) / serum albumin ratio and found increased BBB permeability in SE and BBB dysfunction occurring more often in acute symptomatic SE11, 12. However, this method cannot measure the integrity of BBB of a specific brain area.This explorative study measured BBB permeability in several brain areas of NORSE patients for the first time, aiming to characterize BBB dysfunction in NORSE using DCE-MRI to provide preliminary evidence on its specificity and pathogenesis.Methods
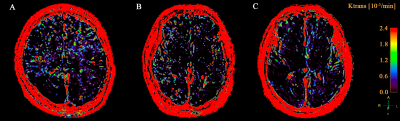
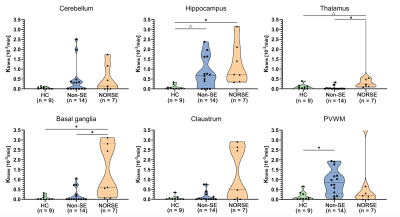
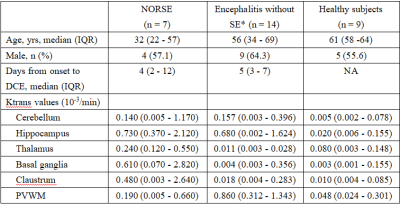
This study included three groups of adult participants: patients with NORSE, encephalitis patients without status epilepticus (SE), and healthy subjects. These participants were retrospectively included from a prospective DCE-MRI database of neurocritically ill patients and healthy subjects. The BBB permeability (Ktrans) in the hippocampus, basal ganglia, thalamus, claustrum, periventricular white matter (PVWM), and cerebellum were measured and compared between these three groups.Results
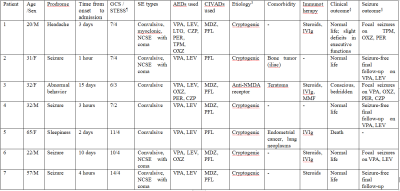
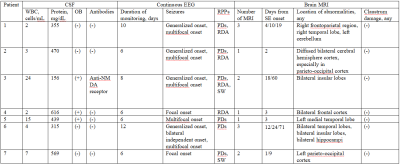
A total of 7 patients with NORSE, 14 encephalitis patients without SE, and 9 healthy subjects were included in this study. Among 7 patients with NORSE, only one person had a definite etiology (autoimmune encephalitis), the rest were cryptogenic. Etiology of encephalitis patients without SE included viral (n = 2), bacterial (n=8), tuberculous (n = 1), cryptococcal (n = 1), and cryptic (n = 2) encephalitis. Of these 14 encephalitis patients without SE, 3 patients had seizures. Compared to healthy controls, NORSE patients had increased Ktrans values in all selected regions, with significant differences in the hippocampus (0.73 vs 0.02 Í 10-3/min, p = 0.001) and basal ganglia (0.61 vs 0.003 Í 10-3/min, p = 0.007) and a significant trend in the thalamus (0.24 vs 0.08 Í 10-3/min, p = 0.017). Compared to encephalitis patients without SE, NORSE patients had increased Ktrans values in all selected regions except cerebellum and PVWM, with significant differences in the thalamus (0.24 vs 0.01Í 10-3/min, p = 0.002) and basal ganglia (0.61 vs 0.004 Í 10-3/min, p = 0.013).Discussion
In this explorative study, we investigated the BBB dysfunction in six brain areas of 7 patients with NORSE and compared their BBB permeability with that of encephalitis patients without SE and healthy subjects. This study demonstrated that BBBs of NORSE patients were impaired diffusely, and they had significantly higher BBB permeability in the basal ganglia and thalamus compared to encephalitis patients without SE. Our preliminary findings underscore the important role of basal ganglia and thalamus in the development of NORSE.Conclusion
This explorative study demonstrates that BBBs of NORSE patients were impaired diffusely, and BBB dysfunction in the basal ganglia and thalamus plays an important role in the pathophysiology of NORSE.Acknowledgements
Not applicable.References
1. Sutter R, Kaplan PW, Ruegg S. Independent external validation of the status epilepticus severity score. Crit Care Med 2013;41: e475-479.
2. Aukland P, Lando M, Vilholm O, Christiansen EB, Beier CP. Predictive value of the Status Epilepticus Severity Score (STESS) and its components for long-term survival. BMC Neurol 2016; 16:213.
3. Giovannini G, Monti G, Polisi MM, et al. A one-year prospective study of refractory status epilepticus in Modena, Italy. Epilepsy Behav 2015;49: 141-145.
4. Yuan F, Yang F, Jia R, et al. Multimodal Predictions of Super-Refractory Status Epilepticus and Outcome in Status Epilepticus Due to Acute Encephalitis. Front Neurol 2018;9: 832.
5. Brophy GM, Bell R, Claassen J, et al. Guidelines for the evaluation and management of status epilepticus. Neurocrit Care 2012;17: 3-23
6. Nicolas Gaspard, Brandon P Foreman, Vincent Alvare, et al. New-onset refractory status epilepticus Etiology, clinical features, and outcome. Neurology 2015;85(18):1604-13.
7. Matthews E, Alkhachroum A, Massad N, et al. New-onset super-refractory status epilepticus: A case series of 26 patients. Neurology 2020;95: e2280-e2285.
8. Heye AK, Culling RD, Valdes Hernandez Mdel C, Thrippleton MJ, Wardlaw JM. Assessment of blood-brain barrier disruption using dynamic contrast-enhanced MRI. A systematic review. Neuroimage Clin 2014;6: 262-274.
9. Kersten Villringer, Borja E Sanz Cuesta, Ann-Christin Ostwaldt, et al. DCE-MRI blood-brain barrier assessment in acute ischemic stroke. Neurology 2017;88(5):433-440.
10. Cramer SP, Simonsen H, Frederiksen JL, Rostrup E, Larsson HB. Abnormal blood-brain barrier permeability in normal appearing white matter in multiple sclerosis investigated by MRI. Neuroimage Clin 2014;4: 182-189.
11.J Correale, A L Rabinowicz, C N Hec, et al. Status epilepticus increases CSF levels of neuron-specific enolase and alters the blood- brain barrier. Neurology 1998;50(5):1388-91.
12. Malter MP, Choi S, Fink GR. Cerebrospinal fluid findings in non-infectious status epilepticus. Epilepsy Res 2018;140: 61-65.
Figures