5347
Reduced white matter axonal density in bipolar disorder using Neurite Orientation Dispersion and Density Imaging (NODDI)
Gail I. S. Harmata1, Hesam Abdolmotalleby1, John E. Barsotti1, Jess G. Fiedorowicz1,2, Aislinn Williams1, Gary Christensen1, Jia Xu1, Joseph J. Shaffer1,3, Jeffrey D. Long1, Jenny Gringer Richards1, Leela Sathyaputri1, Samantha L. Schmitz1,4, John A. Wemmie1, Vincent A. Magnotta1, and Merry Mani1
1University of Iowa, Iowa City, IA, United States, 2University of Ottawa, Ottawa, ON, Canada, 3Kansas City University, Kansas City, MO, United States, 4Des Moines University, Des Moines, IA, United States
1University of Iowa, Iowa City, IA, United States, 2University of Ottawa, Ottawa, ON, Canada, 3Kansas City University, Kansas City, MO, United States, 4Des Moines University, Des Moines, IA, United States
Synopsis
Keywords: Psychiatric Disorders, White Matter, bipolar disorder
Bipolar disorder is a serious psychiatric condition whose cause remains unknown. Previous diffusion MRI studies suggest white matter alterations may be involved, but standard diffusion tensor scalars provide limited information regarding potential pathophysiology. Here we used Neurite Orientation Dispersion and Density Imaging (NODDI) to examine select white matter bundles to provide additional information regarding changes in microstructure. We found that the uncinate fasciculus, cingulum hippocampus, and corpus callosum genu showed evidence of reduced axonal density, suggesting axonal loss or altered neurodevelopment. Additional work is necessary to determine how this pattern changes over time, and how it relates to mood lability.Introduction
Bipolar disorder (BD) is a serious mental health condition characterized by episodes of abnormal mood. Although bipolar disorder has been linked to a variety of changes in brain structure and function, the underlying pathophysiology of bipolar disorder remains unknown. Meta-analyses of diffusion imaging studies have reported alterations in fractional anisotropy (FA) in bipolar disorder1-3, suggesting that changes in white matter could be leading to altered brain connectivity. However, traditional diffusion imaging techniques such as diffusion tensor imaging do not provide detailed information about white matter microstructure, which is critical for understanding the changes occurring in bipolar disorder. An alternative modeling approach called Neurite Orientation Dispersion and Density Imaging (NODDI)4 may provide better specificity to detected signal changes by relating it to specific tissue microstructural properties. Specifically, NODDI assumes a three-compartment model consisting of an intra-neurite, extra-neurite, and free-water compartment. Fitting the NODDI model provides two primary scalars: the neurite dispersion index (NDI) and the orientation dispersion index (ODI). Unlike FA derived from the diffusion tensor, NDI and ODI separates the density and dispersion of axonal bundles in a voxel into two independent parameters and thus provide more specific microstructural information. NODDI has previously been employed in bipolar disorder research in a few studies5-7, but has not been used to examine white matter tracts specifically. Therefore, we used NODDI to investigate white matter tracts previously implicated in emotional processing and/or bipolar disorder.Methods
For this study we recruited control participants and participants with bipolar disorder type I who all provided written informed consent before enrolling into the study. All procedures were IRB-approved. Participants underwent a multi-modal imaging study on a 3T MRI scanner. Imaging included acquisition of volumetric T1 and T2 weighted scans along with a multi-shell diffusion imaging protocol with the following parameters: TE=88ms, TR=10s, FOV=256x256mm, Matrix=128x128, # diffusion directions = 60 per shell, and b-values = 1000 and 1800s/mm2. Five b0 images with reversed polarity phase encoding were collected for distortion correction. BRAINS tools8 were used to automatically analyze the anatomical images and define a corresponding brain mask. The diffusion images were corrected for geometric distortions and corrected for eddy-current artifacts. Gibbs ringing correction and Rician bias correction was performed using DESIGNER9. NODDI fitting was performed using the Microstructure Diffusion Toolbox10,11. 3dSkullStrip from AFNI12 was used to extract the brain on the diffusion b0 images and ANTS13 was used to provide a mapping from the diffusion weighted images to the anatomical images. ANTS deformable registration was then used to map the Mori white matter atlas14 onto the anatomical images for each participant. NODDI diffusion scalars were assessed in each of the fiber tracts. We selected 9 white matter tracts of interest based on prior literature1,3,15 to examine for differences in NODDI diffusion metrics: corpus callosum body, splenium, and genu; left and right uncinate fasciculi; left and right cingulum cingulate gyrus; and left and right cingulum hippocampal gyrus. We were specifically interested in the NODDI metrics neurite dispersion index (NDI), Dperp, and orientation dispersion index (ODI); these metrics relate to neurite density, extra-axonal radial diffusivity, and fiber orientation dispersion, respectively. Some participants were unable to complete all brain scans, and 6 scans were excluded after manual review and quality control screening. The resulting dataset used for statistical analysis included 77 control participants and 124 participants with bipolar disorder. Using regression analysis in R/RStudio16,17, we then tested for differences between control participants and participants with bipolar disorder while controlling for age and sex. We corrected for multiple comparisons for each metric using false discovery rate (FDR).Results
We found that NDI was reduced and Dperp was elevated in bipolar disorder for the cingulum hippocampus bilaterally, the uncinate fasciculus bilaterally, and the corpus callosum genu (FDR q-value < 0.05). This indicates that participants with bipolar disorder tend to have reduced axonal density in these regions, suggesting axons may have been lost over time or did not develop normally. This is consistent with fractional anisotropy reductions previously reported in these white matter bundles1-3. Additionally, ODI was trending lower in bipolar disorder in the cingulum hippocampus bilaterally after FDR correction but did not reach statistical significance. This trend could also be consistent with selective reduction of axons in a region with high orientation dispersion in controls18, and may also be consistent with a previous report that found reduced ODI in the left hippocampus in bipolar disorder6.Conclusion
Our results suggest that reduced axonal density in white matter in emotion-related brain networks may be an important feature in bipolar disorder. Additional longitudinal studies are needed to determine the nature of this reduction and how it relates to changes in mood. Future work should also examine whether these metrics are sensitive to medications such as lithium. Overall, this work extends previous literature on diffusion imaging in bipolar disorder and demonstrates how more advanced modeling techniques can be employed to make advances in psychiatric research.Acknowledgements
This study was supported by funding from the National Institute of Mental Health (R01MH111578, T32MH019113) and the Iowa Neuroscience Institute, with studies conducted on equipment (S10OD025025) and facilities (UL1TR002537) supported by NIH.References
- Benedetti F, Absinta M, Rocca MA, et al. Tract-specific white matter structural disruption in patients with bipolar disorder. Bipolar Disorders. 2011;13(4):414-424. doi:https://doi.org/10.1111/j.1399-5618.2011.00938.x
- Vederine F-E, Wessa M, Leboyer M, Houenou J. A meta-analysis of whole-brain diffusion tensor imaging studies in bipolar disorder. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2011/12/01/ 2011;35(8):1820-1826. doi:https://doi.org/10.1016/j.pnpbp.2011.05.009
- Yang C, Li L, Hu X, et al. Psychoradiologic abnormalities of white matter in patients with bipolar disorder: diffusion tensor imaging studies using tract-based spatial statistics. J Psychiatry Neurosci. Jan 1 2019;44(1):32-44. doi:10.1503/jpn.170221
- Zhang H, Schneider T, Wheeler-Kingshott CA, Alexander DC. NODDI: Practical in vivo neurite orientation dispersion and density imaging of the human brain. NeuroImage. 2012/07/16/ 2012;61(4):1000-1016. doi:https://doi.org/10.1016/j.neuroimage.2012.03.072
- Nazeri A, Mulsant BH, Rajji TK, et al. Gray Matter Neuritic Microstructure Deficits in Schizophrenia and Bipolar Disorder. Biological Psychiatry. 2017/11/15/ 2017;82(10):726-736. doi:https://doi.org/10.1016/j.biopsych.2016.12.005
- Ota M, Noda T, Sato N, et al. The use of diffusional kurtosis imaging and neurite orientation dispersion and density imaging of the brain in bipolar disorder. Journal of Affective Disorders. 2019/05/15/ 2019;251:231-234. doi:https://doi.org/10.1016/j.jad.2019.03.068
- Sarrazin S, Poupon C, Teillac A, et al. Higher in vivo Cortical Intracellular Volume Fraction Associated with Lithium Therapy in Bipolar Disorder: A Multicenter NODDI Study. Psychotherapy and Psychosomatics. 2019;88(3):171-176. doi:10.1159/000498854
- Pierson R, Johnson H, Harris G, et al. Fully automated analysis using BRAINS: AutoWorkup. Neuroimage. Jan 1 2011;54(1):328-36. doi:10.1016/j.neuroimage.2010.06.047
- Ades-Aron B, Veraart J, Kochunov P, et al. Evaluation of the accuracy and precision of the diffusion parameter EStImation with Gibbs and NoisE removal pipeline. Neuroimage. Dec 2018;183:532-543. doi:10.1016/j.neuroimage.2018.07.066
- Harms RL, Fritz FJ, Tobisch A, Goebel R, Roebroeck A. Robust and fast nonlinear optimization of diffusion MRI microstructure models. Neuroimage. Jul 15 2017;155:82-96. doi:10.1016/j.neuroimage.2017.04.064
- Harms RL, Roebroeck A. Robust and Fast Markov Chain Monte Carlo Sampling of Diffusion MRI Microstructure Models. Original Research. Frontiers in Neuroinformatics. 2018-December-18 2018;12doi:10.3389/fninf.2018.00097
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. Jun 1996;29(3):162-73. doi:10.1006/cbmr.1996.0014
- Avants BB, Tustison NJ, Song G, Cook PA, Klein A, Gee JC. A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage. Feb 1 2011;54(3):2033-44. doi:10.1016/j.neuroimage.2010.09.025
- Mori S, Oishi K, Jiang H, et al. Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. NeuroImage. 2008/04/01/ 2008;40(2):570-582. doi:https://doi.org/10.1016/j.neuroimage.2007.12.035
- van der Horn HJ, Mangina NR, Rakers SE, et al. White matter microstructure of the neural emotion regulation circuitry in mild traumatic brain injury. https://doi.org/10.1111/ejn.15199. European Journal of Neuroscience. 2021/05/01 2021;53(10):3463-3475. doi:https://doi.org/10.1111/ejn.15199
- R: A language and environment for statistical computing. R Foundation for Statistical Computing; 2021. https://www.R-project.org/
- RStudio: Integrated Development Environment for R. RStudio, PBC; 2020. http://www.rstudio.com/
- Kamiya K, Hori M, Aoki S. NODDI in clinical research. Journal of Neuroscience Methods. 2020/12/01/ 2020;346:108908. doi:https://doi.org/10.1016/j.jneumeth.2020.108908
Figures
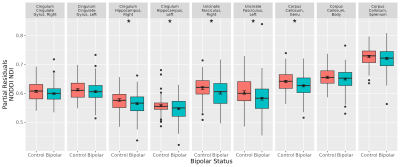
Figure 1. Regression analysis of NODDI neurite dispersion index (NDI) metric for the bipolar disorder and control groups (N = 124 and 77, respectively), after controlling for age and sex. Cingulum hippocampus right and left, uncinate fasciculus right and left, and corpus callosum genu were reduced in bipolar disorder (*FDR q-value < 0.05). Boxplot shows median (crossbar), interquartile range (box), range (line), and outliers (points). Also shown are the mean +/- standard error (small dash with error bars at center).
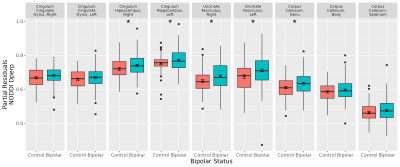
Figure 2. Differences in bipolar
disorder in NODDI Dperp, controlling for age and sex (N Control Group = 77, N Bipolar Group = 124). Cingulum hippocampus right and left, uncinate
fasciculus right and left, and corpus callosum genu were elevated in bipolar
disorder (*FDR q-value < 0.05).
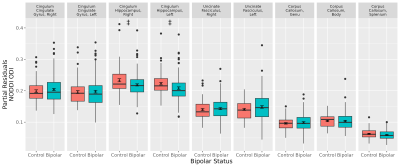
Figure 3. Differences in bipolar disorder in NODDI orientation dispersion index (ODI), controlling for age and sex (N Control Group = 77, N Bipolar Group = 124). Cingulum hippocampus right and left were trending lower in bipolar disorder (‡FDR q-value < 0.1) but did not reach statistical significance.
DOI: https://doi.org/10.58530/2023/5347