5345
7-Tesla in-vivo 1H-magnetic resonance spectroscopy of glutamate and GABA in 22q11.2 copy number variants.
Chaira Serrarens1, Desmond HY Tse2, Esther Steijvers-Peeters2, Kim Brouwers2, David Linden1, Claudia Vingerhoets1, and Therese van Amelsvoort1
1Department of Psychiatry & Neuropsychology, School for Mental Health and Neuroscience, Maastricht University, Maastricht, Netherlands, 2Scannexus BV, Maastricht, Netherlands
1Department of Psychiatry & Neuropsychology, School for Mental Health and Neuroscience, Maastricht University, Maastricht, Netherlands, 2Scannexus BV, Maastricht, Netherlands
Synopsis
Keywords: Psychiatric Disorders, Spectroscopy, Genetic Diseases
22q11.2 copy number variants (22q11.2 CNVs) are associated with either an increased or a reduced risk of developing psychotic disorders and impaired cognitive functioning. Glutamatergic and GABAergic pathways are hypothesized to be disrupted in 22q11.2 CNV patients. Although a balance between glutamate and GABA is necessary for optimal brain functioning, to date, GABA has not been studied in 22q11.2 CNVs. Here, we investigated glutamate and GABA concentrations in the anterior cingulate cortex in patients with 22q11.2 CNVs using 7-Tesla 1H-MRS. Our results showed no significant differences in glutamate and GABA concentrations between 22q11.2 CNV patients and healthy controls.Introduction
22q11.2 copy number variants (22q11.2 CNVs) are genetic disorders caused by a microdeletion (22q11.2DEL) or microduplication (22q11.2DUP) at chromosome 22. 22q11.2DEL individuals are at increased risk of developing psychotic disorders and impaired cognitive functioning (1), while 22q11.2DUP individuals are at reduced risk of developing psychotic disorders (2). Psychosis and cognitive impairments have been linked with glutamatergic dysregulation (3). Increased hippocampal glutamate and Glx concentrations have previously been found in 22q11.2DEL patients with schizophrenia compared with 22q11.2DEL patients without schizophrenia (4). Yet, other studies did not show alterations in anterior cingulate cortex (ACC) and striatal glutamate and Glx levels in 22q11.2DEL patients compared with healthy controls (5,6). To date, glutamatergic function has not been studied in 22q11.2DUP individuals. Glutamate function is closely correlated with GABA, an inhibitory neurotransmitter that has also been implicated in psychosis and cognition (7). Although a balance between glutamate and GABA is necessary for optimal brain functioning, to date, GABA has not been studied in 22q11.2 CNVs. Research into the glutamate/GABA balance in 22q11.2 CNVs may lead to possible targets for pharmacological treatment. Here, we aimed to investigate alterations in glutamate and GABA concentrations in the ACC in patients with 22q11.2 CNVs.Methods
Eight 22q11.2DEL patients (mean age = 36.75; M/F = 3/5; mean IQ = 81.63) and 3 22q11.2 duplication syndrome (22q11.2DUP) patients (mean age = 32.67; M/F = 2/1; mean IQ = 100.67) without a history of psychiatric illness and 14 matched healthy controls (mean age = 30.71; M/F = 7/7; mean IQ = 109.36) were enrolled in this study. All MR measurements were performed on a MAGNETOM 7T MR scanner (Siemens Healthineers, Erlangen, Germany) using a single- channel transmit/32-channel receive head coil (Nova Medical, Wilmington, MA, USA). Anatomical (T1-weighted) images were acquired using a magnetization-prepared two rapid acquisition gradient-echo (MP2RAGE (8)) sequence: TR/TE = 4500/2.39 ms, TI1/TI2 = 900/2750 ms, flip angle = 5°/3°; voxel size = 0.9 mm isotropic, matrix size = 256 × 256 × 192, phase partial Fourier = 6/8, GRAPPA factor = 3 with 24 reference lines, bandwidth = 250 Hz/pixel, acquisition time = 6:00 min. These anatomical images were used to guide the manual positioning of the voxel at the ACC (Figure 1). Glutamate spectra (Figure 2a) were acquired using a stimulated echo acquisition mode (STEAM (9)) sequence: TR/TE/TM = 5000/6.0/10.0 ms, NA = 64, flip angle = 90°, voxel size = 25 x 20 x 17 mm3, RF bandwidth = 4.69 kHz, RF centered at 2.4 ppm, receive bandwidth = 4kHz, vector size = 2048, 16-step phase cycling, acquisition time = 5:20 min. GABA spectra (Figure 2b) were acquired using a Mescher- Garwood-semi-localised by adiabatic selective refocusing (MEGA-sLASER (10)) sequence: TR/TE= 7500/74.0 ms, voxel size = 25 x 30 x 30 mm3, NA= 64, editing pulse between 1.9 (edit-on) and 1.5 ppm (edit-off), receive bandwidth = 4kHz, vector size = 2048, acquisition time = 16:55 min. Water suppression was performed using VAPOR (11). A complete phase cycle of measurements was acquired without the water suppression RF pulses to record a water peak reference for eddy current correction (12) and absolute metabolite concentration calibration (13,14). Before the spectroscopy measurements, a 3D-GRE dual-echo field-map (TE1 = 1.00 ms, TE2 = 2.98 ms, TR = 20.0 ms, flip angle = 8°, voxel size = 3 mm isotropic, matrix size = 84 × 84 × 56, bandwidth = 1450 Hz/pixel, acquisition time = 2:24 min) was acquired and used to calculate the shim currents required to homogenize the static magnetic field within the spectroscopic voxels of interest. Glutamate spectra were analyzed with LCModel version 6.3-1L (15) using a GAMMA-simulated basis set (16). GABA spectra were analyzed with Gannet version 3.1 (17) implemented in MATLAB. Spectral quality measures, including signal-to-noise ratio (SNR), Cramèr–Rao lower bound (CRLB) and linewidth of glutamate, and fit error of GABA, were calculated and addressed for differences between groups (Table 1). Tissue probability maps for grey matter (GM), white matter (WM) and cerebrospinal fluid (CSF) were generated from the T1- weighted images using FSL-FAST. GM, WM and CSF partial volumes within the spectroscopy voxels were estimated from these tissue probability maps. Glutamate and GABA concentrations were corrected for the proportion of CSF (18).Results
We did not find significant differences in glutamate concentrations between groups (F(2,21) = 0.657; p = 0.528; ƞ2 = 0.059; Figure 3). In addition, we did not find significant differences in GABA concentrations between groups (F(2,13) = 0.592; p = 0.567; ƞ2 = 0.083; Figure 4).Discussion and conclusion
Using separate optimized sequences for the quantification of glutamate and GABA, significant alterations in ACC metabolite concentrations in 22q11.2 CNVs were not found. These findings are in line with previous studies in 22q11.2DEL patients, showing no glutamatergic alterations (5,6). Given that our sample size was relatively small, resulting in decreased power to detect statistically significant differences, we cannot exclude the possibility of an altered glutamate/GABA balance in 22q11.2 CNVs. However, data collection for this study is still ongoing. More and larger studies are required to replicate these findings in 22q11.2 CNVs.Acknowledgements
We would like to thank all subjects for participating in this study. We would also like to thank Pandi Veeraiah for his help scanning participants, Nele Volbragt for study coordination, and Nele Soons, Sophie Kappert and Jeltje Spapens for helping with recruitment.
References
1. Vorstman JAS, Breetvelt EJ, Duijff SN, Eliez S, Schneider M, Jalbrzikowski M, et al. Cognitive decline preceding the onset of psychosis in patients with 22q11.2 deletion syndrome. JAMA Psychiatry. 2015;72(4):377–85.2. Rees E, Kirov G, Sanders A, Walters JTR, Chambert KD, Shi J, et al. Evidence that duplications of 22q11. 2 protect against schizophrenia. 2014;(September 2013):37–40.
3. Bojesen KB, Broberg BV, Fagerlund B, Jessen K, Thomas MB, Sigvard A, et al. Associations Between Cognitive Function and Levels of Glutamatergic Metabolites and Gamma-Aminobutyric Acid in Antipsychotic-Naïve Patients With Schizophrenia or Psychosis. Biol Psychiatry [Internet]. 2021;89(3):278–87. Available from: https://doi.org/10.1016/j.biopsych.2020.06.027
4. Alves S, Boot E, Schmitz N, Nederveen A, Vorstman J, Pouwels P, et al. Proton Magnetic Resonance Spectroscopy in 22q11 Deletion Syndrome. 2011;6(6).
5. Rogdaki M, Hathway P, Gudbrandsen M, McCutcheon RA, Jauhar S, Daly E, et al. Glutamatergic function in a genetic high-risk group for psychosis: A proton magnetic resonance spectroscopy study in individuals with 22q11.2 deletion. Eur Neuropsychopharmacol. 2019;29(12):1333–42.
6. Vingerhoets C, Tse DHY, van Oudenaren M, Hernaus D, van Duin E, Zinkstok J, et al. Glutamatergic and GABAergic reactivity and cognition in 22q11.2 deletion syndrome and healthy volunteers: A randomized double-blind 7-Tesla pharmacological MRS study. J Psychopharmacol. 2020;34(8):856–63.
7. Taylor SF, Tso IF. GABA abnormalities in schizophrenia: A methodological review of in vivo studies. Schizophr Res Author. 2015;167(0):84–90.
8. Marques JP, Kober T, Krueger G, van der Zwaag W, Van de Moortele PF, Gruetter R. MP2RAGE, a self bias-field corrected sequence for improved segmentation and T1-mapping at high field. Neuroimage [Internet]. 2010;49(2):1271–81. Available from: http://dx.doi.org/10.1016/j.neuroimage.2009.10.002
9. Frahm J, Merboldt KD, Hänicke W. Localized proton spectroscopy using stimulated echoes. J Magn Reson. 1987;72(3):502–8.
10. Andreychenko A, Boer VO, Arteaga De Castro CS, Luijten PR, Klomp DWJ. Efficient spectral editing at 7 T: GABA detection with MEGA-sLASER. Magn Reson Med. 2012;68(4):1018–25.
11. Tkáč I, Starčuk Z, Choi IY, Gruetter R. In vivo 1H NMR spectroscopy of rat brain at 1 ms echo time. Magn Reson Med. 1999;41(4):649–56.
12. Klose U. In vivo proton spectroscopy in presence of eddy currents. Magn Reson Med. 1990;14(1):26–30.
13. Barker PB, Soher BJ, Blackband SJ, Chatham JC, Mathews VP, Bryan RN. Quantitation of proton NMR spectra of the human brain using tissue water as an internal concentration reference. NMR Biomed. 1993;6(1):89–94.
14. Soher BJ, Hurd RE, Sailasuta N, Barker PB. Quantitation of automated single-voxel proton MRS using cerebral water as an internal reference. Magn Reson Med. 1996;36(3):335–9.
15. Provencher SW. Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed. 2001;14(4):260–4.
16. Smith SA, Levante TO, Meier BH, Ernst RR. Computer Simulations in Magnetic Resonance. An Object-Oriented Programming Approach. Vol. 106, Journal of Magnetic Resonance, Series A. 1994. p. 75–105.
17. Edden RAE, Puts NAJ, Harris AD, Barker PB, Evans CJ. Gannet: a batch-processing tool for the quantitative analysis of GABA-edited MRS spectra. J Magn Reson Imag. 2011;4(164):1445–52.
18. Quadrelli S, Mountford C, Ramadan S. Hitchhiker’S Guide to Voxel Segmentation for Partial Volume Correction of in Vivo Magnetic Resonance Spectroscopy. Magn Reson Insights. 2016;9:MRI.S32903.
Figures
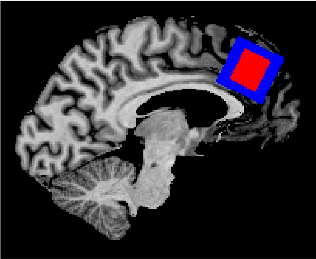
Figure1. ACC voxel positioning. The blue box indicates the location of the ACC as measured with the MEGA-sLASER sequence. The red box indicates the location the ACC as measured with the STEAM sequence.
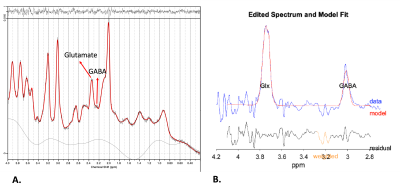
Figure 2. (A) Example of the ACC spectrum acquired using STEAM. (B) Example of the ACC spectrum acquired using MEGA-sLASER.
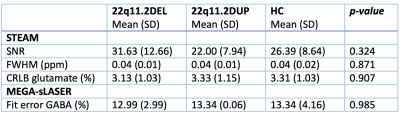
Table 1. Spectral quality of included subjects.
SNR: signal-to-noise ratio; FWHM: full width at half maximum; ppm: parts per million; CRLB: Cramèr–Rao lower bound; HC: healthy control.
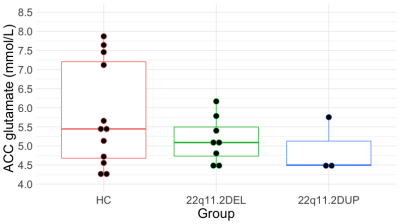
Figure 3. ACC Glutamate concentrations in healthy controls (HC) and individuals with 22q11.2DEL and 22q11.2DUP as measured with the STEAM sequence.
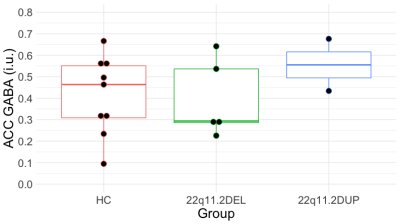
Figure 4. ACC GABA concentrations in healthy controls (HC) and individuals with 22q11.2DEL and 22q11.2DUP as measured with the MEGA-sLASER sequence.
DOI: https://doi.org/10.58530/2023/5345