5337
Neuropsychiatric systemic lupus erythematosus is associated with a distinct type and cerebral structural changes1the First Hospital of China Medical University, shenyang, China
Synopsis
Keywords: Gray Matter, Neuroinflammation, NPSLE
We used the voxel-based morphometry (VBM) to investigate morphological alterations within gray matter (GM) in patients with neuropsychiatric systemic lupus (NPSLE). The two subgroups (inflammatory and ischemic phenotypes) have different pathological mechanisms and treatment methods. In the comparison of grey matter volumes within the two patient groups, we found brain atrophy was more severe in the inflammatory group and that the atrophied brain areas were consistent with previous studies of the blood-brain barrier in patients with autoimmune disease. Our findings could help to deeply understand the pathophysiology of NPSLE, and to treat lupus patients early to improve their prognosis.
Methods: Participants: Seventy-four patients ( 39 NPSLE including 22 inflammatory and 17 ischemic phenotypes) were randomly recruited from The First Hospital of China Medical University, fulfilling at least four of the American College of Rheumatology (ACR) classification criteria for SLE. 28 age and sex-matched healthy controls (HCs) were enrolled in community.
Data acquisition and preprocessing: MRI scan was performed on the 3.0T pioneer GE MRI. Three-dimensional T1-weighted images were acquired in a sagittal orientation employing a Sag bravo sequence: repetition time (TR)=7.8 ms, echo time (TE)=3.0 ms, field of view (FOV)=24 cm×24 cm, matrix=240×240, slice thickness=1.0 mm, slices=176, no gap. For each participant, the MRI scanning lasted 4.5 min. the segmented and normalized gray matter (GM) images in MNI space were modulated and smoothed for the voxel-based morphometry (VBM) analysis toolbox in SPM based on the MATLAB platform.
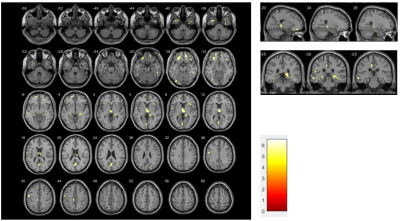
Results: Differences in whole brain grey matter volume between NPSLE patients and healthy controls: Two-sample t-tests were performed to observe the differences in whole brain grey matter volume between the two groups. Compared to HCs, patients with NPSLE presented widespread GM atrophy in the cortical cortex including the frontal (e.g. rectus, orbitofrontal cortex, middle/inferior frontal gyrus), temporal (e.g. superior/inferior temporal gyrus), parietal (e.g. precuneus) and occipital (e.g. fusiform, inferior occipital gyrus) cortex, subcortical nuclei (e.g. thalamus, hippocampus) (Figure 1).
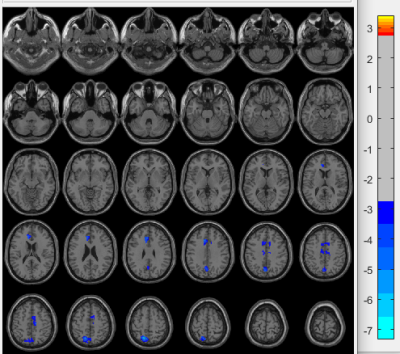
Changed grey matter volumes between two NPLSE subgroups: Compared to the inflammatory group, patients with ischemic phenotype presented GM atrophy, GM atrophy mainly located in the cortical cortex including the middle cingulate which belongs to the limbic lobe, cuneus and precuneus (Figure 2).
Discussion:In this study, we investigated abnormalities of grey matter volumes hypothesized to be affected in NPSLE and studied their associations between two subtypes and cognitive functions[3]. One of our main findings was a reduction of grey matter volume in all tracts investigated in the SLE group when compared to HC, albeit it was significantly decreased in the frontal lobe and thalamus, which come from the prefrontal loop and keep in close connection with the thalamic medial part. Precuneus is an important component of the default mood network (DMN),where abnormalities are often found in functional MRI studies of previous lupus patients. Previous studies have shown evidence of hippocampus atrophy in patients with lupus, including blood-brain barrier disruption in these regions, Anti-neural antibody production (NMDAR,neuronal surface P antigen) [4]. All the factors indicate that the grey matter microstructure in SLE patients is altered in areas crucial for attention, mood, and cognitive functions.
In the subgroup of lupus patients, brain atrophy was more severe in the inflammatory group than in the ischemic one, probably because of the different pathological mechanisms between them, predominantly inflammatory-neurotoxic one mediated by complement activation, increased
permeability of the BBB, but the other mediated by aPL, immune complexes, and leuko-agglutination. Most of our results focus on the limbic system and midline cortical structure, especially the cingulate gyrus region, which is around the lateral ventricle and is vulnerable to blood-brain barrier disruption. The parietal is a part of the posterior circulation system supplied by the vertebrobasilar artery, unlike the frontoparietal cortex, which is supplied by the anterior circulation. It appears that a relative lack of sympathetic innervation of the posterior circulation promotes vulnerability or diminished cerebral autoregulation, and ultimately causes increased BBB leakage[5].
Conclusion: In conclusion, we found that NPSLE patients have significant grey matter atrophy compared to HCs, and different subgroups of NPSLE have different atrophy modes. These results imply that structural research could improve our understanding of the pathophysiology of NPSLE.
Acknowledgements
I would like to thank my teachers and fellow students for their guidance and helpReferences
[1] Govoni M, Bortoluzzi A, Padovan M, Silvagni E, Borrelli M, Donelli F, Ceruti S, Trotta F. The diagnosis and clinical management of the neuropsychiatric manifestations of lupus. J Autoimmun. 2016 Nov;74:41-72.
[2] Kamintsky L, Beyea SD, Fisk JD, Hashmi JA, Omisade A, Calkin C, Bardouille T, Bowen C, Quraan M, Mitnitski A, Matheson K, Friedman A, Hanly JG. Blood-brain barrier leakage in systemic lupus erythematosus is associated with gray matter loss and cognitive impairment. Ann Rheum Dis. 2020 Dec;79(12):1580-1587.
[3] Rolls ET. The cingulate cortex and limbic systems for emotion, action, and memory. Brain Struct Funct. 2019 Dec;224(9):3001-3018.
[4] Schwarting A, Möckel T, Lütgendorf F, Triantafyllias K, Grella S, Boedecker S, Weinmann A, Meineck M, Sommer C, Schermuly I, Fellgiebel A, Luessi F, Weinmann-Menke J. Fatigue in SLE: diagnostic and pathogenic impact of anti-N-methyl-D-aspartate receptor (NMDAR) autoantibodies. Ann Rheum Dis. 2019 Sep;78(9):1226-1234.
[5] Moon Y, Lim C, Kim Y, Moon WJ. Sex-Related Differences in Regional Blood-Brain Barrier Integrity in Non-Demented Elderly Subjects. Int J Mol Sci. 2021 Mar 11;22(6):2860.