5333
Prediction meningioma grade by constructing a cli-radiomics model nomogram based on magnetic resonance imaging1Lanzhou University Second Hospital, LanZhou, China
Synopsis
Keywords: Tumors, Machine Learning/Artificial Intelligence
This study investigate the feasibility of a cli-radiomics model in preoperative noninvasive prediction of meningioma grade. This study attempted to construct a radiomics model for predicting meningioma grade based on T1C and T2WI sequences using different classifiers, select the optimal radiomics model, and combine it with clinical labels to construct a nomogram. Decision curve analysis was used to verify the clinical validity of the nomogram, which provides a non-invasive and convenient alternative method for clinicians to predict meningioma grades.Introduction
Introduction: Meningiomas are the most common brain tumors of meningeal origin, accounting for 39.0 percent of intracranial tumors1. The World Health Organization classification of central nervous system tumors divides meningiomas into three grades and fifteen subtypes2. WHO grade I meningiomas account for 81% of all meningiomas and have a 10-year overall survival rate of over 90%. The incidence of WHO grade III meningiomas is low, but the recurrence and mortality rates are extremely high, with a 10-year overall survival rate of 33%3. The prognosis of meningiomas is influenced by many factors4, such as pathological grade, consistency, degree of surgical resection, brain invasion, and bone invasion. Follow-up observation or radiotherapy is the primary treatment for early and small-sized low-grade meningiomas (LGM). High-grade meningiomas (HGM) are often difficult to completely resect and have a high rate of invasion and recurrence. Surgical resection combined with chemoradiotherapy is the primary treatment5. Therefore, preoperative non-invasive and accurate prediction of LGM and HGM is helpful for the selection of individualized therapy and reduces the financial burden on patients. Some conventional MRI signs of different grades of meningiomas overlap and mainly rely on the subjective judgment of radiologists to reflect the size and shape of meningiomas, which lacks of objectivity and stability. Therefore, there is an urgent need for a non-invasive, simple, and accurate method to predict meningioma grade and provide evidence for clinical decision-making. radiomics can objectively and quantitatively capture some valuable pathophysiological information of tumors that are difficult to recognize by the naked eye and evaluate tumor heterogeneity in a non-invasive manner6. Radiomics has been applied in the differential diagnosis, pathological grading and prognosis of meningiomas. Previous studies have focused on radiomics with small sample sizes. The purpose of this study was to construct a nomogram of the cli-radiomics model by fusing clinical factors and radiomics features, and verifying the consistency and clinical validity of the nomogram through calibration curve and decision curve analysis, which can be more intuitive and visual for clinical practice.Methods
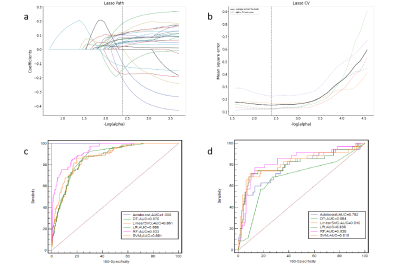
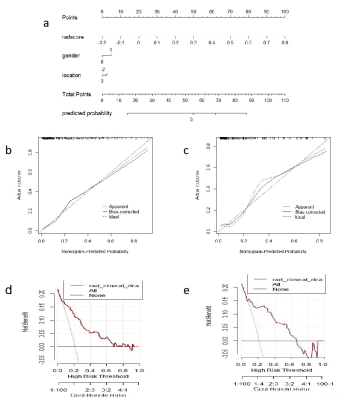
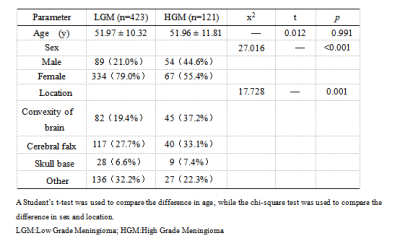
methods: We collected retrospectively 544 patients with pathological diagnosis of meningiomas were categorized into training (n=380) and validation (n=164) groups at the ratio of 7∶ 3. There were 3376 radiomics features extracted from T2WI and T1C by shukun.net after manual segmentation using an independent blind method by two radiologists. The Selectpercentile and Lasso are used to filter the most strongly correlated features. Random forests (RF) radiomics model and cli-radiomics model nomogram were constructed respectively. The calibration, discrimination, and clinical validity were evaluated by using the calibration curve and decision analysis (DCA).Results
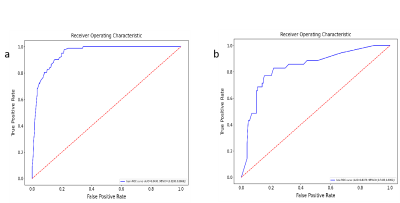
Results: The RF radiomics model based on T1C and T2WI was the most effective to predict meningioma grade before surgery among the six different classifiers. The predictive ability of cli-radiomics model was slightly higher than that of RF model alone. The AUC, SEN, SPE, and ACC of the training set were 0.949, 0.976, 0.785, and 0.826, and the AUC, SEN, SPE, and ACC of the validation set were 0.838, 0.829, 0.783, and 0.793, respectively. The calibration curve and Hosmer-Lemeshow test showed the predictive probability of the fusion model was similar to the actual differentiated LGM and HGM. The analysis of the decision curve showed that the cli-radiomics model could obtain the best clinical net profit.Discussion
Discussion: The WHO classification of meningiomas is a key determinant for predicting tumor recurrence and overall survival and helps guide the development of postoperative radiotherapy and follow-up strategies [16,17]. The results suggest that twenty-five radiomics features were selected to construct radiomics models by six classifiers, and RF was superior to other prediction models in diagnostic efficiency in the training and verification sets. Finally, we found the cli-radiomics model that was constructed using the random forest and clinical features (sex and location) was the best. The AUC values of the training and validation sets were significant at 0.949 and 0.838, respectively. The calibration curve and Hosmer-Lemeshow test further confirmed no significant difference between the results of differentiating LGMs from HGMs and those predicted by the cli-radiomics model (p=0.086 and 0.143, respectively). Decision curve analysis assessed the clinical availability of cli-radiomics models and provided the greatest net clinical benefit for preoperative prediction in patients with HGMs.Conclusion
Conclusions: The cli-radiomics model nomogram based on T1C and T2WI has high accuracy and sensitivity for predicting meningioma grade, and it has important clinical value in accurately and conveniently predicting meningioma grade preoperatively.Acknowledgements
We are greatly indebted to all patients, doctors, and statistical consultants who were involved in our study.References
1. Ostrom, QT., Cioffi, G., Waite, K., et al., CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2014-2018[J]. Neuro Oncol, 2021. 23(12 Suppl 2): p. iii1-iii105.
2. Zhang, T., Yu, J. M., Wang, Y. Q., et al., WHO grade I meningioma subtypes: MRI features and pathological analysis[J]. Life Sci, 2018. 213: p. 50-56.
3. Kim, L., A narrative review of targeted therapies in meningioma[J]. Chin Clin Oncol, 2020. 9(6): p. 76. 4. Sauvigny, T., Ricklefs, F. L., Hoffmann, L.et al., Features of tumor texture influence surgery and outcome in intracranialmeningioma[J]. Neurooncol Adv, 2020. 2(1): p. vdaa113.
5. Yang, L., Xu P., Zhang Y., et al., A deep learning radiomics model may help to improve the prediction performance of preoperative grading in meningioma. Neuroradiology, 2022;64(7):1373-82.
6. Lambin, P., Rios-Velazquez, E., Leijenaar, R., et al., Radiomics: extracting more information from medical images using advanced feature analysis[J]. Eur J Cancer, 2012. 48(4): p. 441-6.
Figures