5332
Reproducibility of 13C labeling of glutamate and glutamine in human brain using selPOCE MRS at 7T upon [U-13C]-labeled glucose infusion1Radiology, University Medical Center Utrecht, Utrecht, Netherlands, 2Neurosurgery, University Medical Center Utrecht, Utrecht, Netherlands, 3Yale University School of Medicine, New Haven, CT, United States, 4MSD (Europe) Inc, Brussel, Belgium, 5Merck & Co. Inc, Kenilworth, NJ, United States, 6ICON plc, Groningen, Netherlands
Synopsis
Keywords: Non-Proton, Metabolism, 13C
The aim of this study is to assess the reproducibility of a selective proton-observed, carbon-edited (selPOCE) MRS sequences for the detection of glutamate/glutamine cycling at 7T in healthy participants. Participants were scanned twice while undergoing [U-13C] glucose infusion. The time course of Glu C45 and Gln C45 labeling were similar for test-retest measurements. This supports the application in future studies on measuring neuroenergetics and neurotransmitter cycling.Introduction
Glutamate is the major excitatory neurotransmitter in the central nervous system1. During the glutamate/glutamine (Glu/Gln) neurotransmitter cycle, excitatory glutamatergic neurons can take up glucose and oxidize it via the tricarboxylic acid (TCA) cycle2-4.Glu/Gln neurotransmitter cycling in the brain can be measured by the detection of 13C labeling of Glu and Gln in the brain during the infusion of 13C-labeled glucose using 13C magnetic resonance spectroscopy (MRS)2-4. While the sensitivity of 13C MRS is low, it can be enhanced using indirect detection using spectral editing. Compared to direct 13C MRS, indirect 13C MRS has a lower spectral resolution and Glu and Gln are less well resolved. The latter can be overcome by using selective proton-observed, carbon-edited (selPOCE) MRS5.
Monitoring Glu/Gln metabolism may provide important biomarkers for studies on various brain diseases. The reproducibility of determining this metric using selPOCE MRS in humans is not yet known. Here we assess the reproducibility of selPOCE MRS for detecting of 13C-labeled Glu and Gln at 7T in the brain.
Methods
Experimental protocolFour healthy male adults (age:23 ± 2.2 years) underwent 1H-[13C]) MRS in a 7T MR-system (Philips, Best, NL) on two occasions, at least 3 weeks apart. A 13C transmit birdcage head coil, combined with 8 transmit-receive 1H dipole antennas and a 32-channel 1H receive array (Nova Medical, Wilmington, USA) was used6. One participant was excluded due to significant head movements. Participants were fasted overnight. After IV-catheter placement in both arms, participants were positioned in the MR system. T1-weighted images were acquired for voxel positioning and B0 and B1 shimming in a manually drawn ROI in the frontal lobe was performed. The infusion protocol started with an initial, exponential bolus of 99% enriched [U-13C] glucose lasting 20 min, followed by 100 min of continuous infusion of 66% enriched [U-13C] glucose and 33% unlabeled glucose, allowing the concentration of plasma glucose to fall slowly while maintaining the fractional enrichment (FE) constant.
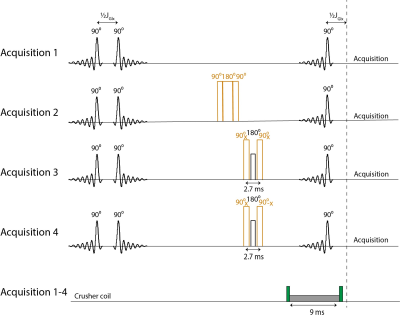
A selPOCE STEAM MRS sequence was used for dynamic (6:40 min scan interval) detection of the H4 of Glu and Gln signals, labeled at carbons 4 and 5 (referred to as Glu C45 and Gln C45) in a voxel in the frontal lobe (minimal voxel size 3x5x3 cm; TE 7.8ms; TM 30ms; TR 3000ms; NSA 4x32; VAPOR water suppression). The selPOCE STEAM sequence consisted of four acquisitions: (1) conventional STEAM; (2) 13C inversion, using a composite 13C 90°-180°-90° block pulse set to 32.95 ppm, i.e. in between the C4 resonances of Glu and Gln; (3 and 4) 13C Glu and Gln phase discrimination achieved with 13C 90°(+x)-1H 180°-13C 90°(+/-x) block pulses, also set to 32.95 ppm (figure 1). A crusher coil was used to suppress residual signals from extracranial lipids.
Blood samples were collected every 5 minutes to determine plasma glucose levels using a benchtop glucose analyzer (YSI® 2500 series) and for later determination of 13C FE of plasma glucose by 1H MRS at 500 MHz.
Post-Processing
Coil combination was performed using a generalized-least-squares algorithm, using the unsuppressed-water signal to compute the coil sensitivity of each channel. Spectra were frequency and phase aligned, and subtracted per fourfold for selPOCE7. The Glu C45, Gln C45 and Glx C3 signals in the difference spectra were fitted with a home-written LCModel approach in MATLAB (MathWorks, USA).
The time course of Glu C45 and Gln C45 signals were normalized to the average amplitude of Glu C45 of the final ~30 minutes of each experiment (steady state).
The test/retest variability of Glu C45 and Gln C45 was defined as: $$ Variability(T/RT)= \frac{R_{\frac{Early}{SS}_{Retest}} - R_{\frac{Early}{SS}_{Test}}}{(R_{\frac{Early}{SS}_{Retest}}+R_{\frac{Early}{SS}_{Test}})/2} $$
Where REarly/SS is the Glu C45 (or Gln C45) signal over early time points (i.e. ~20-45 min after start infusion) normalized to the Glu C45 signal during steady state.
Results
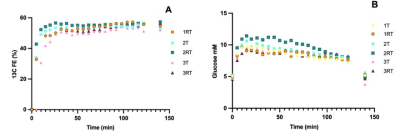
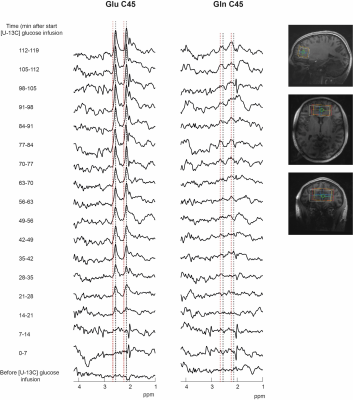
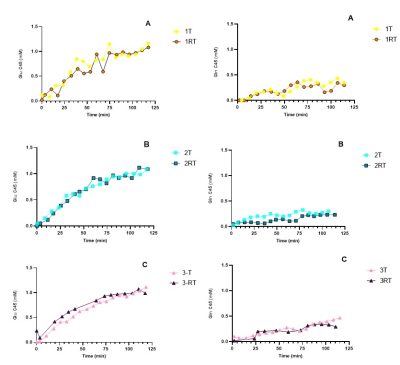
There was a rapid increase of plasma glucose 13C FE and plasma glucose concentrations during the first 20 min of infusion, followed by a steady state of 13C FE until the end of the infusion. The time courses were similar for T/RT (Figure 2). FE of plasma glucose in participant 1-Test and the first datapoints of participant 3-Retest could not be determined, due to insufficient water suppression.Figure 3 shows the selPOCE difference spectra from one volunteer. The Glu C45 and Gln C45 signals can be observed, as well as the overlapping resonances of Glx C3. The time-dependent increase of Glu C45 and Gln C45 signals for all participants can be seen in figure 4. As expected, labeling of Glu C45 was faster compared to Gln C45 labeling and the steady-state ratio Glu C45 / Gln C45 was on average 0.31±0.06.
REarly/SS for Glu test and retest was similar (mean 0.51±005 vs. 0.52±0.01) with a test/retest variability of 9.7%. For Gln REarly/SS test and retest was similar as well (0.16±0.02 vs. 0.13±0.04); however, test/retest variability was higher compared to Glu (32.4%).
Discussion and conclusion
We have investigated the reproducibility of selPOCE MRS at 7T for detection of labeled Glu and Gln. Time courses of 13C labeling of Glu and Gln labeling were similar for test retest. Knowing the variability of these readings supports the application to future studies on for example disease specific alterations in Glu/Gln cycling.Acknowledgements
No acknowledgement found.References
1. Sarlo GL, Holton KF. Brain concentrations of glutamate and GABA in human epilepsy: A review. European Journal of Epilepsy. 2021;91(1):213–27.
2. Sibson NR, Mason GF, Shen J, Cline GW, Herskovits AZ, Wall JE, Behar KL, Rothman DL,Shulman RG. In vivo (13)C NMR measurement of neurotransmitter glutamate cycling, anaplerosisand TCA cycle flux in rat brain during. J Neurochem. 2001;76(4):975–989.
3. An L, Li S, Ferraris Araneta M, Johnson CS, Shen J. Detection of 13C labeling of glutamate and glutamine in human brain by proton magnetic resonance spectroscopy. Sci Rep. 2022;12(1):1–9.
4. Mason GF, Gruetter R, Rothman DL, Behar KL, Shulman RG, Novotny EJ. Simultaneous determination of the rates of the TCA cycle, glucose utilization, α-ketoglutarate/glutamate exchange, and glutamine synthesis in human brain by NMR. Journal of Cerebral Blood Flow & Metabolism. 1995;15(1):12-25.
5. De Feyter, H. M. et al. Selective proton-observed, carbon-edited (selPOCE) MRS method for measurement of glutamate and glutamine C-13-labeling in the human frontal cortex. Magn. Reson. Med. 80, 11–20.
6. Jacobs SM, Prompers JJ, van der Kemp WJM, van der Velden TA, Gosselink WJM, Hoogduin JM, Mason GF, de Graaf RA, van der Kolk AG, Alborahal C, Klomp DW, Wiegers EC. Human brain POCE MRS at 7T using a 13C birdcage coil and 8 transmit-receive 1H antennas with a 32-channel 1H receive array. Proceedings of the Joint ISMRM-ESMRMB 31st Annual Meeting.
7 Rothman DL, de Feyter HM, de Graaf RA, Mason GF, Behar KL. 13C MRS studies of neuroenergetics and neurotransmitter cycling in humans. NMR Biomed. 2011;24(8):943–57.
Figures