5330
129Xe sublimation DNP towards hyperpolarized imaging standards1Laboratory of Functional and Metabolic Imaging, EPFL, Lausanne, Switzerland, 2HYPERMAG, Technical University of Denmark, Copenhagen, Denmark
Synopsis
Keywords: Hyperpolarized MR (Gas), Hybrid & Novel Systems Technology
Sample preparation of 129Xe for dDNP with different glassing solvents, radical choice and concentrations affects the electron properties as well as the overall achieved 129Xe polarization. A short T1e and/or poor mixing is improved by ad hoc solutions that results in competitive polarization values in solid state measurements.
The sample preparation of 129Xe for DNP showed an improved incorporation of the gas atoms in the solvent when using ultrasonication. Applying microwave frequency modulation during the irradiation reached a higher 129Xe polarization. Extracted Xe was measured in a 1T NMR benchtop spectrometer to be 18s.
INTRODUCTION
Hyperpolarized 129Xe MRI is widely used in clinics to investigate lung diseases and investigate perfusion of the brain tissues1,2. Hyperpolarization of the gas is achieved using the well-established method known as spin exchange optical pumping (SEOP)3.Dissolution Dynamic Nuclear Polarization (dDNP)4 is the other clinically available hyperpolarization technique. It enables following in real time the fate of 13C-labelled molecules, mainly used to investigate pathologies entailing abnormal metabolism5. Differently from SEOP, DNP’s strongest point is versatility: in theory, all NMR active nuclei can be hyperpolarized in the solid state via DNP, thus also 129Xe. Having one hyperpolarization device that can cover both clinically relevant nuclei would be practically and financially advantageous. It has been demonstrated that dDNP, followed by sublimation, can produce preclinical doses of 129Xe gas hyperpolarized up to 10%6,7. Although encouraging, the current sublimation DNP state-of-the art is still far from what can be achieved with 13C-labelled molecules, and one of the main reasons is the choice of a suitable glassing matrix that host well-mixed solid Xe and radicals. The latter obliges us to use unusual solvents for DNP (i.e. short chain alcohol) that are still liquid when the temperature approaches the Xe triple point.
ESR characterization of the radical/polarizing agent is crucial to achieve efficient DNP and consequently high substrate polarization8. In this study we investigate the properties of two widely used radicals for DNP when dissolved in three unusual glassing solvents by LOngitudinalDetected-ESR (LOD-ESR). Finally, on one sample formulation, we show the clear advantage of using microwave frequency modulation and cryogenic temperature ultrasonication.
MATERIALS AND METHODS
LOD-ESR:different concentrations of TEMPO (2,2,6,6-Tetramethyl-1-piperidinyloxy) and “Finland trityl” (tris(8-carboxy-2,2,6,6-tetramethylbenzo[1,2-d:4,5-d′]-bis(1,3dithiol-4-yl) methyl sodium salt) were dissolved into ethanol, 2-Methyl-1-propanol (isobutanol) and 2-Methyl-1-pentanol (pentanol). Droplets were flash frozen in liquid nitrogen and loaded into the LOD-ESR spectrometer as earlier described9. The T1e and ESR spectrums were measured at 5T/1.2 K.
DNP:
A TEMPO concentration of 30mM after dissolution in isobutanol and Xe incorporation. Firstly, Xe gas was added atop the solution. Secondly, immersing the sample vial in an ethanol bath (i.e. -110°C), mixing the biphasic liquid manually or by ultrasonication (500W/20kHz/10 min). The sample vial was then connected to a custom fluid path (CFP)2 and loaded into the 5T/1.2K polarizer. Microwave frequency sweeps were collected for both samples without/with 100MHz microwave frequency modulation (FM), determining optimal DNP conditions.
Sublimation DNP experiment:
The sample providing the highest enhancement was fully polarized and dissolved with D2O (7.5mL, 180°C). Xe gas was phase separated by the mist of hot solvent and He gas used to blow out from the polarizer the hyperpolarized solution. The gas was then trapped in a 5mm NMR tube placed inside a 1.05T benchtop NMR spectrometer (SpinSolve 43 13C/129Xe, Magritek) measuring signal decay of the hyperpolarized gas with 5° pulses every 4.5s.
RESULTS AND DISCUSSION
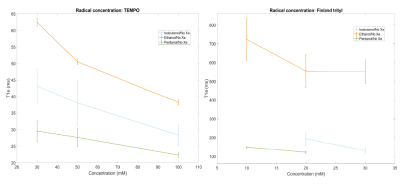
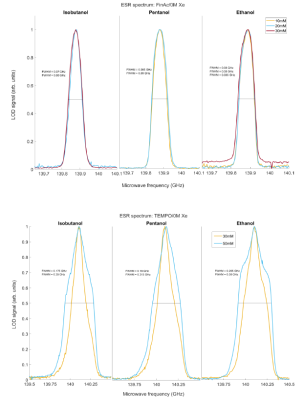
We report the T1e measurements (Figure 1). The first striking feature is the difference in values of the electron spin-lattice relaxation time as a function of the type of solvent. In the case of TEMPO at the lowest concentration, we observed 62±2 ms when using ethanol, and only 30±3 ms for 2-Methyl-1-pentanol. The T1e for each solvent decreases as a function of increasing radical concentration by a factor 1.5. It is remarkable to notice that all these values are much shorter compared to nitroxyl radicals, at same experimental conditions, dissolved in the glycerol:water, has relaxation times 150-200 ms10. Most likely, in the different matrixes the phonon spectral density is very different11, which also applies for trityl. In the solvents, besides ethanol, the relaxation time is approximately 5 times shorter compared to the same radical concentration in standard 13C-labelled DNP samples9.Remarkably, the influence of radical concentration on the ESR spectral width (Figure 2): trityl nearly unaffected (FWHM ~85 MHz), but doubling the TEMPO concentration makes the line-width 50% broader, a major influence of the dipolar coupling.
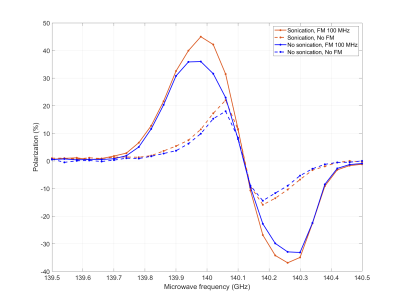
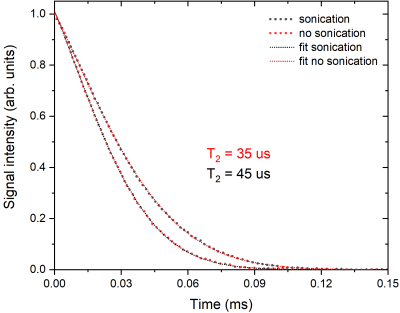
Concerning DNP, we tackled problems of mixing the Xe well into the matrix and the surprisingly short T1e by using ultrasonication and microwave FM, respectively. We report the influence of the two techniques (Figure 3) on the DNP enhancement as a function of irradiation frequency. Cryogenic ultrasonication improves the homogenization of the sample, confirmed by the longer T2 (Figure 4), leading to more efficient polarization transfer. Microwave frequency modulation improved spectral diffusion and engages more electron spin in the DNP mechanism.
At optimal conditions, 129Xe could be polarized up to 40% (Figure 3). When maximum polarization was achieved, the sample was dissolved and the Xe gas extracted. The T1 value of 18s (Figure 5) at room temperature is shorter than expected, probably because of solvent residues after sublimation.
CONCLUSIONS AND PERSPECTIVES
We have demonstrated that Xe DNP is a challenging topic due to the multiple phase transitions involved in the sample preparation and subsequent extraction of the gas. Nevertheless, the short T1e and intrinsic poor mixing between substrate and polarizing matrix can be solved using ad hoc solutions to obtain competitive polarization values in the solid state. We foresee to improve the extraction process to shelter the polarization and retain enough signal for advanced hyperpolarized imaging sequences.Acknowledgements
No acknowledgement found.References
| [1] | M. R. Rao, N. J. Stewart, P. D. Griffiths, G. Norquay and J. M. Wild, "Imaging human brain perfusion with inhaled hyperpolarized 129Xe MR imaging," Radiology, vol. 286, no. 2, pp. 659-665, 2018. |
| [2] | J. T. Grist, G. J. Collier, H. Walters, M. Kim, M. Chen, G. A. Eid, A. Laws, V. Matthews, K. Jacob, S. Cross, A. Eves, M. Durant, A. Mcintyre, R. Thompson, R. F. Schulte, B. Raman, P. A. Robbins, J. M. Wild, E. Fraser and F. Gleeson, "Lung Abnormalities Depicted with Hyperpolarized Xenon MRI in Patients with Long COVID," Radiology, p. 220069, 2022. |
| [3] | T. G. Walker and W. Happer, "Spin-exchange optical pumping of noble-gas nuclei," Rev. Mod. Phys, vol. 69, no. 2, pp. 629-642, 1997. |
| [4] | K. Golman, R. Zandt and M. Thaning, "Real-time metabolic imaging," Proceedings of the National Academy of Sciences, vol. 103, no. 30, pp. 11270-11275, 2006. |
| [5] | J. Kurhanewicz, D. Vigneron, J. Ardenkjaer-Larsen, J. Bankson, K. Brindle, C. Cunningham, F. Gallagher, K. Keshari, A. Kjaer, C. Laustsen, D. Mankoff, M. Merritt, S. Nelson, J. Pauly, P. Lee, S. Ronen, D. Tyler, S. Rajan, D. Spielman, L. Wald, X. Zhang, C. Malloy and R. Rizi, "Hyperpolarized 13C MRI: Path to Clinical Translation in Oncology," Neoplasia, vol. 1, no. 16, 2019. |
| [6] | A. Comment, S. Jannin, J.-N. Hyacinthe,, P. Miéville, R. Sarkar, P. Ahuja, P. R. Vasos, X. Montet, F. Lazeyras, J.-P. Vallée, P. Hautle, J. A. Konter, B. van den Brandt, J.-P. Ansermet, R. Gruetter and G. Bodenhausen, "Hyperpolarizing Gases via Dynamic Nuclear Polarization and Sublimation," Physical Review Letters, vol. 105, no. 018104 , 2010. |
| [7] | A. Capozzi, C. Roussel, A. Comment and J.-N. Hyacinthe, "Optimal Glass-Forming Solvent Brings Sublimation Dynamic Nuclear Polarization to 129Xe Hyperpolarization Biomedical Imaging Standards," The Journal of Physical Chemistry, pp. 5020-5025, 2015. |
| [8] | L. Lumata, Z. Kovacs, A. D. Sherry, C. Malloy, S. Hill, J. Van Tol, L. Yu, L. Song and M. E. Merritt, "Electron spin resonance studies of trityl OX063 at a concentration optimal for DNP," Physical Chemistry Chemical Physics, vol. 15, no. 24, pp. 9800-9807, 2013. |
| [9] | T. Phong Lê, J.-N. Hyacinthe and A. Capozzi, "How to improve the efficiency of a traditional dissolution dynamic nuclear polarization (dDNP) apparatus: Design and performance of a fluid path compatible dDNP/LOD-ESR probe," Journal of Magnetic Resonance, vol. 338, no. 107197, 2022. |
| [10] | A. Capozzi, A. Kilund, M. Karlsson, S. Patel, A. C. Pinon, F. Vibert, O. Ouari, M. H. Lerche and J. Ardenkjær-Larsen , "Metabolic contrast agents produced from transported solid 13C-glucose hyperpolarized via dynamic nuclear polarization.," Commun Chem, vol. 4, no. 95, 2021. |
| [11] | A. Capozzi, S. Patel, W. T. Wenckebach, M. Karlsson, M. H. Lerche and J. H. Ardenkjær-Larsen, "Gadolinium effect at high-magnetic-field DNP: 70\% 13C polarization of [U-13C] glucose using trityl," The journal of physical chemistry letters, vol. 10, no. 12, pp. 3420-3425, 2019. |
Figures
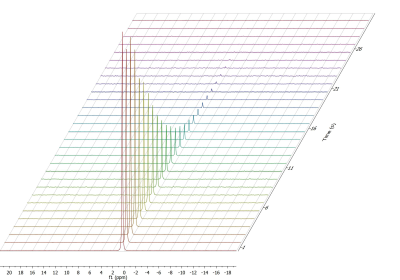
Gas-state HP 129Xe signal at 1.05T after sublimation and extraction. The T1 was 18.4s.