5325
Feasibility of a multimodal MR Imaging and Spectroscopy approach in understanding Myalgic Encephalomyelitis/Chronic Fatigue Syndrome
Raminder Kaur1,2, Kashish Mehta1,2, Alexander Ciok1,2, Brian Greeley1, Kati Debelic3, Hilary Robertson3, Todd Nelson1,2, Melody Tsai4, Lan Xin Zhang1,5, Margit Glashutter1,2, Travis Boulter4, Luis Nacul*4,6, and Xiaowei Song*1,2
1Clinical Research, Fraser Health Authority, Surrey, BC, Canada, 2Department of Biomedical Physiology Kinesiology, Simon Fraser University, Burnaby, BC, Canada, 3Patient partner in Research, Community member, Vancouver, BC, Canada, 4BC Women's Hospital & Health Centre, Vancouver, BC, Canada, 5Medical Biophysics, University of Toronto, Toronto, ON, Canada, 6University of British Columbia, Vancouver, BC, Canada
1Clinical Research, Fraser Health Authority, Surrey, BC, Canada, 2Department of Biomedical Physiology Kinesiology, Simon Fraser University, Burnaby, BC, Canada, 3Patient partner in Research, Community member, Vancouver, BC, Canada, 4BC Women's Hospital & Health Centre, Vancouver, BC, Canada, 5Medical Biophysics, University of Toronto, Toronto, ON, Canada, 6University of British Columbia, Vancouver, BC, Canada
Synopsis
Keywords: Head & Neck/ENT, Multimodal
This first multimodal MRI/MRS study aiming to investigate whether an extensive multimodal neuroimaging approach is feasible for patients with ME/CFS. The study compared female ME/CFS participants to aged matched female healthy controls. The study showed successful completion of the entire protocol that included: anatomical imaging; functional magnetic resonance imaging (fMRI) along with a working memory task; single-voxel magnetic resonance spectroscopy (MRS) to quantify metabolites at three locations (ACC, BS, and l-DLPC); and a hand-grip strength to observe whether fatigue is induced due to the scanning protocol. It also suggested a minimum impact of the MRI session on grip strength.Introduction
Myalgia Encephalomyelitis, also known as chronic fatigue syndrome (ME/CFS) is a multi-facetted disorder resulting in neurological dysfunction causing long-term debilitating symptoms including persistent fatigue.1-4 Predominantly affecting women, the disorder impacts over 580,000 Canadians and up to 24 million people worldwide.1 With a lack of understanding and no effective treatment for ME/CFS, patients generally have a low quality of life.3-4 To better understand the underlying neural mechanisms of ME/CFS, we conducted a multimodal MRI / MRS study. This pilot study compared female ME/CFS participants to aged-matched female healthy controls (HC). The protocol included anatomical and functional magnetic resonance imaging (fMRI) along with a working memory task, single-voxel magnetic resonance spectroscopy (MRS) at multiple locations to quantify metabolites, and a hand-grip strength test. To our knowledge, this multimodal approach has not been explored to understand ME/CFS.Objectives
This pilot study aimed to investigate whether the multimodal neuroimaging protocol is feasible for patients with ME/CFS. Specifically, whether the ME/CFS patients could complete the MR protocol. We also explored whether it is possible to detect a difference in brain functional, metabolic, and anatomical MRI/MRS and other data collected in the study between the patient and control participants.Methods
Eighteen female ME/CFS and five HC participants were enrolled in the study from the Complex Chronic Diseases Program and community, respectively. All participants provided informed consent. Eligibility criteria for the ME/CFS group included: meeting diagnostic criteria of Canadian Consensus Criteria or Institute of Medicine; no significant co-morbidity, a fatigue analog score <5, fatigue severity score >36, Generalised Anxiety Disorder-2 score <4, and a physical health questionnaire-2 score <4 at the time of their Pre-Screening Survey. Eligibility criteria for control participants included females aged 19-69 years; the absence of co-morbidities that implicate the presence of fatigue or cognitive difficulties.Participants completed the fMRI (with visual N-back working memory tasks), MRS, and a hand-grip strength test immediately before and after the neuroimaging protocol [Figure 1], conducted at the SFU ImageTech Lab situated in the Surrey Memorial Hospital of Fraser Health using a Philips Ingenia 3.0 T system equipped with a 32-channel dStream headcoil. Scans were acquired by MR technologists who practiced the protocol for acquiring high-quality data. The total scan time was one hour with sequence time being approximately 40 minutes.
Three single voxels (SV) MRS included voxels of interest (ROI) being placed over the anterior cingulate cortex (ACC), brainstem (BS), and the left dorsolateral prefrontal cortex (l-DLPFC). These provided spectral data of multiple metabolites including N-acetylaspartate (NAA), Creatine (CR), and phosphocholine (PCh) [Figure 2].
With fMRI, participants were asked to perform the N-back task using letter stimuli with two levels of difficulty, matching a series of letters either 1- or 2-back during the fMRI portion of the scan [Figure 3].
Artifacts including motion and susceptibility were visually reviewed to assess the data quality before further processing and analysis. fMRI data processing followed standard procedures using FSL (FMRIB's Software Library, www.fmrib.ox.ac.uk/fsl).5-7 MRS analysis was performed following standard pipelines using LCModel.8 Response accuracy and delay time performing fMRI tasks and grip strength data were also examined, using descriptive statistics, analyses of variance, and comparison between participant groups.
Results
Study completion: The sample characteristics are presented in Table 1. ME/CFS participants showed a high willingness to research participation with a 100% response rate to the initial invitation to participate in the study and no dropouts. All participants completed the pre-screening surveys, pre-and post-hand-grip strength tests, cognitive tasks with fMRI, and MRI scans of three SVs without interruption or reporting adverse events[Figure 1].MRS data: All participants completed the MRS data collection. The voxels were consistently placed applying anatomical landmarks[Figure 3]. Quantification of the three major metabolites was successfully performed for all participants. Spectra from the BS and the DPLFC were nosier reflecting the tissue boundaries at these locations.
fMRI data: All participants completed the task-state fMRI session, with high quality data supporting both individual level mapping, and group level comparisons of activation and contrast maps.
Task performance data: All participants understood and performed the fMRI tasks without difficulty. A trend of differences between the decision accuracy and response time was observed.
Hand grip strength and impact of the MRI /MRS scan: All ME/CFS participants were able to complete the pre- and post-scan hand grip test. ME/CFS participants showed similar handgrip strength results before (24.86 ±1.8kg) and after (25.21 ±2.2kg) the MRI session, which are somewhat lower than those of HC
Discussion
We successfully conducted a multimodal MRI/MRS study, demonstrating its feasibility for application in studying patients with ME/CFS. The pilot study suggested promising preliminary results showing differences in patient vs control across all data. The success rate in task completion and no dropouts from the ME/CFS participants indicate that research using a multimodal approach is feasible and acceptable for further investigation. Ongoing research by our group will aim to refine data acquisition and analyses and explore group differences with interpretation using the study sample. A better understanding of the changes in the brain in ME/CFS holds promise for advancing the diagnosis, management, and treatment of the medical condition in the future.Acknowledgements
The study receives research funding from BCPOR (G2020-005; G2020-014) and SMF (G2007-001)References
- Shan, ZY, Kwiatek, R, Burnet, R, et al. Medial prefrontal cortex deficits correlate with unrefreshing sleep in patients with chronic fatigue syndrome. NMR in Biomedicine. 2017; 30:e3757. https://doi.org/10.1002/nbm.3757
- van Campen, C., Rowe, P. C., & Visser, F. C. (2018). Blood Volume Status in ME/CFS Correlates with the Presence or Absence of Orthostatic Symptoms: Preliminary Results. Frontiers in Pediatrics, 6, 352. doi:10.3389/fped.2018.00352
- Canadian Community Health Survey Share File, (2018), Statistics Canada. Ontario Ministry of Health and Long-Term Care.
- Pendergrast, T., Brown, A., Sunnquist, M., Jantke, R., Newton, J. L., Strand, E. B., & Jason, L. A. (2016). Housebound versus nonhousebound patients with myalgic encephalomyelitis and chronic fatigue syndrome. Chronic illness, 12(4), 292–307. doi:10.1177/1742395316644770
- Woolrich, M. W., Ripley, B. D., Brady, M., & Smith, S. M. (2001). Temporal Autocorrelation in Univariate Linear Modeling of FMRI Data. NeuroImage, 14(6), 1370–1386. http://doi.org/10.1006/nimg.2001.0931
- Woolrich, M. W., Behrens, T. E. J., & Smith, S. M. (2004). Constrained linear basis sets for HRF modelling using Variational Bayes. NeuroImage, 21(4), 1748–1761. http://doi.org/10.1016/j.neuroimage.2003.12.024
- Woolrich, M. W., Behrens, T. E. J., Beckmann, C. F., Jenkinson, M., & Smith, S. M. (2004). Multilevel linear modelling for FMRI group analysis using Bayesian inference. NeuroImage, 21(4), 1732–1747. http://doi.org/10.1016/j.neuroimage.2003.12.023
- S.W. Provencher Estimation of metabolite concentrations from localized in vivo proton NMR spectra, Magn. Reson. Med. 30, 672–679 (1993).
Figures
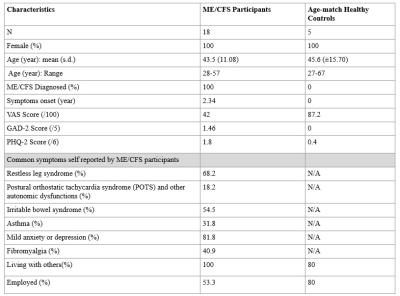
Table 1: Demographics
data of the participants of the study
GAS: Generalised Anxiety Score 2-item; PHQ-2: Patient Health Questionnaire; VAS: Visual Analogue Scale
GAS: Generalised Anxiety Score 2-item; PHQ-2: Patient Health Questionnaire; VAS: Visual Analogue Scale
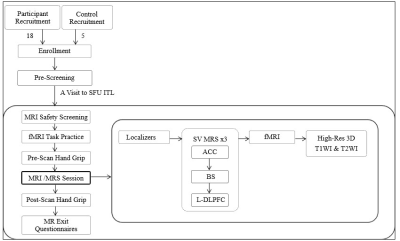
Figure
1: Flow chart summarising participant enrollment/recruitment and MR sequences
participants underwent for the duration of MRI visit.
ACC: anterior cingulate cortex; BS: brainstem; fMRI: functional MRI; L-DLPFC: Left-dorsolateral prefrontal cortex; SFU ITL: Simon Fraser University Image Tech Lab
ACC: anterior cingulate cortex; BS: brainstem; fMRI: functional MRI; L-DLPFC: Left-dorsolateral prefrontal cortex; SFU ITL: Simon Fraser University Image Tech Lab
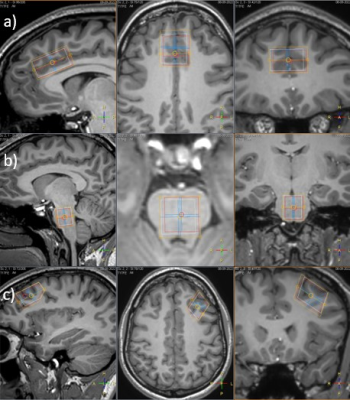
Figure
2: Single voxel placement for MRS at the sagittal plane (left), axial plane
(middle), and coronal plane (right): a) voxel placement at the ACC; placed
along the superior aspect of the corpus callosum at the genu and along the
midline of the sagittal sinus: b) voxel placement at brainstem: voxel aimed to
be placed covering as much as homogenous tissue; c) voxel placement at DLPFC:
placed at the left hemisphere; covering homogenous gray matter
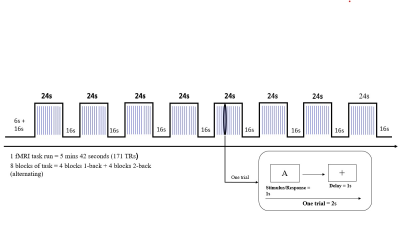
Figure
3: N-Back cognitive task design blocks with activation phase (24s) and rest
phase (16s). The activation phase contained 12 trials followed by rest.
DOI: https://doi.org/10.58530/2023/5325