5320
Characterizing the Network Effects of Deep Brain Stimulation for Obsessive-Compulsive Disorder using Structural and Functional Imaging1Psychiatry, University of California San Francisco, Vallejo, CA, United States, 2Radiology, University of California San Francisco, San Francisco, CA, United States, 3Psychiatry, University of California San Francisco, San Francisco, CA, United States
Synopsis
Keywords: Psychiatric Disorders, Multimodal, brain stimulation
Deep brain stimulation (DBS) is a treatment for severe, refractory OCD. We conducted post-operative DTI imaging to characterize structural connectivity from DBS electrodes. We also conducted fMRI during stimulation ON/OFF studies to elucidate the impact of DBS on functional networks in therapeutic and non-therapeutic configurations. In preliminary analysis, we find that therapeutic contacts are structurally connected to components of the OCD circuit in the anterior cingulate cortex (ACC), caudate, and thalamus. We also identified suppression in this cortico-striato-thalamo-cortical circuit during therapeutic DBS ON vs OFF, suggesting DBS therapy operates by inhibiting the OCD network.Synopsis
Deep brain stimulation (DBS) is a treatment for severe, refractory OCD. We conducted post-operative DTI imaging to characterize structural connectivity from DBS electrodes. We also conducted fMRI during stimulation ON/OFF studies to elucidate the impact of DBS on functional networks in therapeutic configurations. In preliminary analysis, we find that therapeutic contacts are structurally connected to components of the OCD circuit in the anterior cingulate cortex (ACC), caudate, and thalamus. We also identified suppression in this cortico-striato-thalamo-cortical circuit during therapeutic DBS ON vs OFF, suggesting DBS therapy operates by inhibiting the OCD network.Introduction
Deep brain stimulation (DBS) is a targeted treatment for severe, refractory cases of OCD1. We developed methods to characterize the structural connectivity from DBS sites using post-operative DTI. We also collected fMRI during stimulation ON/off studies to characterize the immediate impact of DBS on functional networks in therapeutic and non-therapeutic configurations. We hypothesized that the optimal therapeutic targets for DBS would be strongly connected to and cause suppression in the areas most strongly implicated as components of the OCD cortico-striato-thalamo-cortical (CSTC) circuit: the anterior cingulate (ACC), caudate, and thalamus2.Methods
Three subjects were implanted with an MRI-compatible DBS stimulator as part of their clinical treatment for severe, treatment-refractory OCD. One four-contact lead was implanted in each hemisphere targeting the anterior limb of the internal capsule (ALIC) and neighboring bed nucleus of the stria terminalis (BNST). All three subjects showed marked clinical improvement in their OCD symptoms with continuous stimulation.Patients received T1w MRI and CT scans pre- and post-surgically for electrode localization. One subject also received a pre-surgical DTI scan (55 direction HARDI, b=1000). Post-implantation of the DBS device, we collected T1w anatomical and DTI structural scans (29 directions, b=1000), and gradient-echo fMRI data acquired in low-SAR mode with a 32-channel head coil, TR/TE=2s/30ms, voxel size=3.75x3.75x4cm, flip angle=86, and FOV=24cm. During each fMRI run, we selected one of 12 possible bipolar contact configurations to stimulate and set the DBS device to repeatedly cycle stimulation on for one minute, then off for one minute. Each 6-minute long fMRI scan was timed to begin at the start of the off cycle(Fig1A).
Processing
T1 and fMRI data were preprocessed with fMRIprep3, a standard preprocessing pipeline that combines preprocessing tools from ANTs, FSL, FreeSurfer, AFNI, and Nipype. A T1w weighted patient space template was generated from intensity normalized, skull-stripped and co-registered T1w anatomical scans, which was then registered to MNI152NLin2009cASym space. BOLD runs were slice-time corrected and resampled with head-motion correction parameters calculated in relation to a BOLD reference volume. BOLD runs were registered to the T1w subject template and resampled into MNI152NLin2009cAsym space. Independent component analysis (ICA-AROMA) was performed on the preprocessed MNI-space BOLD time-series after removal of non-steady state volumes and spatial smoothing with an isotropic, Gaussian kernel of 6mm FWHM (full-width half-maximum)4.
Components were manually reviewed by two expert raters, and those identified as noise by both raters were removed. Using AFNI, motion outliers (FD>0.2) and additional polynomial drift terms were removed, and ON-off contrasts were generated. Statistical maps of ON-off contrast were thresholded to p<0.001, clusterized (NN, 40 voxels), extracted as masks, registered to DTI space using ANTs5, and binarized.
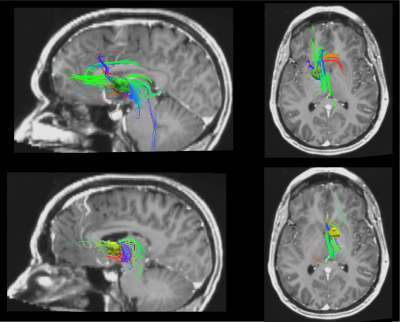
In addition, electrode lead were localized manually in Lead-DBS6 using pre- and postoperative T1w scans. The volume of activated tissue (VAT) for each therapeutic contact was estimated in Lead-DBS, then extracted with MATLAB(Fig1B). VATs were registered to DTI space, dilated by a factor of 10 and binarized. DTI tracts seeded from VATs in Trackvis7 (Fig1C) were visually compared with fMRI ON-off activation and deactivation masks.
Results
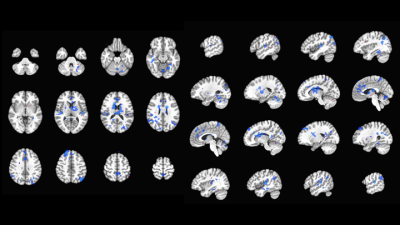
We compared stimulation ON and OFF across subjects for contact pairs that had therapeutic effects (Fig 2). In therapeutic configurations (n=6 runs, 3 subjects), DBS stimulation correlated with BOLD suppression in areas related to OCD, including the bilateral anterior cingulate cortex, caudate, and thalamus. Tracts seeded from electrode VAT ROIs were strongly connected with areas related to OCD network. In all of the therapeutic VAT configurations (n=6 configurations/3 subjects), tracts were found to terminate in the ACC, caudate, insula, and thalamus (Fig3). Five VATs showed tracts terminating in OFC and four in the temporal pole.Discussion
Here, we conducted multi-modal imaging studies to characterize the network impact of DBS for OCD. Using a novel fMRI DBS ON/off paradigm, we identified suppression of BOLD within a network consisting of the ACC, caudate, and thalamus, which are components of the CSTC circuit1. We also identified DTI structural connectivity between active DBS contacts and these regions of the OCD network.Conclusion
Our findings suggest that DBS can relieve OCD symptoms by suppressing key parts of the OCD network1 through structural connections to these regions. This combination of stimulation-based fMRI and structural DTI approach to characterizing the impact of DBS on networks may provide a novel method for optimizing contact locations and parameters to optimally treat severe OCD.Acknowledgements
We would like to acknowledge the generous financial support for this project came from NIMH K23MH125018, Brain Behavior Research Foundation- P&S Fund, Foundation for OCD Research, UCSF RAP GrantReferences
1. Denys, D. et al. Efficacy of Deep Brain Stimulation of the Ventral Anterior Limb of the Internal Capsule for Refractory Obsessive-Compulsive Disorder: A Clinical Cohort of 70 Patients. Am. J. Psychiatry 177, 265–271 (2020).
2. Dougherty, D.D. et al. Neuroscientifically Informed Formulation and Treatment Planning for Patients With Obsessive-Compulsive Disorder. JAMA Psychiatry 75, 1081 (2018).
3. Esteban, O., Markiewicz, C, Blair, R.W., Moodie, C., Isik, A.I., Aliaga, A.E., Kent, J., et al. fMRIPrep: A Robust Preprocessing Pipeline for Functional MRI. Nature Methods (2018).
4. Pruim R.H.R., Mennes M., van Rooij D., Llera A., Buitelaar J.K., Beckmann C.F.. ICA-AROMA: A robust ICA-based strategy for removing motion artifacts from fMRI data. Neuroimage (2015). May 15;112:267-277. doi: 10.1016/j.neuroimage.2015.02.064
5. Avants, B., Tustison, N., & Song, G. Advanced normalization tools (ANTS). Insight J (2008). 1–35. 10.54294/uvnhin.
6. Horn, A., Kühn, A. Lead-DBS: A Toolbox for Deep Brain Stimulation Electrode Localizations and Visualizations. NeuroImage (2014).
7. Wang, R., Benner, T., Sorensen, A.G., Wedeen, V.J. Diffusion Toolkit: A Software Package for Diffusion Imaging Data Processing and Tractography. Proc. Intl. Soc. Mag. Reson. Med. 15 (2007).
Figures