5319
Evaluation of Deep Gray Matter Iron and Myelin Changes in children with Attention-deficit/hyperactivity disorder1First Affiliated Hospital, Sun Yat-sen University, guangzhou, China, 2GE Healthcare, guangzhou, China
Synopsis
Keywords: Neuro, Quantitative Susceptibility mapping
Application of magnetic susceptibility have been consistently demonstrated in the subcortical gray matter of ADHD children, but some uncertainties remain concerning the underlying neurobiological processes. We applied quantitative susceptibility mapping and synthetic magnetic resonance imaging (SyMRI) to clarify the relative contribution of iron and myelin changes to deep gray matter changes in ADHD. We found that the iron and myelin concentration of these subcortical structures in ADHD were delayed in the developmental trajectory, which suggested that ADHD may be characterized by a delay in subcortical maturation and dysfunction in dopaminergic transmission.Introduction
Attention-deficit/hyperactivity disorder (ADHD) is a childhood-onset neuropsychiatric disorder characterized by age-inappropriate inattention and hyperactivity/impulsivity [1]. The etiological bases and neural substrates of childhood ADHD are far from being fully understood. Apart from structure volume decrease [2], several pathologic variations have been demonstrated in the deep gray matter (DGM) of ADHD by means of advanced MRI techniques [3-5]. Among these, recent quantitative susceptibility mapping (QSM) studies explored magnetic susceptibility alterations of subcortical GM, because such changes might reflect iron content and deficiency, which play an important role in ADHD pathophysiology and seem to relate to hyperactivity and inattention issues [4; 5].However, brain magnetic susceptibility is also influenced by other molecules (primarily myelin, quantitatively assessable through the estimation of the method of synthetic MRI[6]), whose spatial distribution remarkably overlaps with iron patterns [7]. The knowledge about the underlying DGM pathophysiological mechanisms in ADHD are not fully understood. In current study, we performed a multimodal (QSM and SyMRI) investigation of DGM, computing in vivo iron- and myelin-specific maps to disentangle the contribution of iron and myelin (concentration and content) abnormalities to subcortical GM in ADHD at regional levels, simultaneously exploring their relationship with clinical variables.Methods
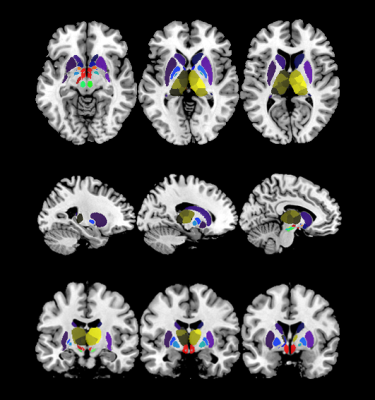
MRI examinations were performed on a 3.0T scanner (SIGNA Pioneer GE Healthcare, WI, USA) using 32-channel head coils. ESWAN sequence was run with 8 echoes to obtain magnitude and phase images: first echo time = 4.4, repetition time = 46.7 ms, flip angle = 20°, field of view = 25.6 × 25.6 cm2, matrix size = 256 × 256, acceleration factor = 2, number of slices = 76, and slice thickness = 2 mm, no gap. Regarding SyMRI, a two-dimensional multiple-dynamic multiple-echo (MDME) pulse sequence, comprising four automatically calculated saturation delay times and two echo times, was applied to acquire the axial sections. The parameters were: TR = 10,205.0 ms, TE = 11.3 ms, flip angle = 20°, thickness = 2 mm/no gap, NEX = 1.00, ETL = 16, pixel size = 2.0 mm × 2.0 mm, scanning time = 5.5 min. Iron- and myelin-concentration maps were derived by QSM- and R1-maps (obtained from SyMRI [6]) by inverting an external affine model estimated in a previous ex-vivo MRI-pathology correlation study at 7-T [7]. The R1-map estimated at 3-T was converted into an expected map at 7-T [8]; susceptibility values were considered independent on magnetic field strength.Forty-eight drug-naïve pediatric ADHD and 41 age-, gender-, and handedness-matched typically developing controls (TDs) were enrolled. Compared with TDs, iron and myelin (concentration and content) of DGM, including subcortical and thalamic nuclei (Figure 1), were assessed with analysis of covariance. Partial correlation analysis was used to assess the relationship between significant MRI parameters and clinical symptom severity in ADHD.
Results
ADHD children showed significant increased iron content in the bilateral putamen and thalamic nuclei and reduced mean iron in bilateral substantia nigra (SN), left parabrachial pigmented nucleus (PBN) and right ventral tegmental area (VTA) (all P < 0.001, Bonferroni-corrected). Regarding myelin, increased myelin concentration was in the right caudate, left internal globus pallidus and bilateral SN, hypothalamus and mamillary nucleus (all P < 0.001, Bonferroni-corrected).For correlation analysis, iron concentration of left substantia nigra pars reticulata (SNpr, r = -0.474, P = 0.0015) and right SNpr (r = -0.349, P = 0.0235) were negatively correlated with clinical symptom severity of ADHD patients.
Discussion
In this study, we evaluated iron and myelin changes to deep gray matter changes in ADHD. The main findings were as follows: 1) increased iron concentration in the bilateral putamen and thalamic nuclei and decreased mean iron in bilateral SN (SNpr and SNpc), left PBN and right VTA were found in ADHD; 2) the increased myelin concentration was mainly at the right caudate, left internal globus pallidus and bilateral SN (SNpr and SNpc), hypothalamus and mamillary nucleus; 3) the iron concentration of bilateral SNpr was positively associate with the clinical symptoms of ADHD.We found that the iron and myelin concentration of these subcortical structures in ADHD were delayed in the developmental trajectory, which suggested that ADHD may be characterized by a delay in subcortical maturation and dysfunction in dopaminergic transmission. Previous studies have already suggested the involvement of the dopaminergic system dysfunction in ADHD [9; 10]. The alterations in dopaminergic systems affect the function of brain structures that moderate executive function, working memory, emotional regulation, and reward processing. We hypothesis that dopaminergic system dysfunction in related DGM regions caused neurobiological alterations.
Conclusion
The subcortical maturation delay and dysfunction in dopaminergic transmission may play an important role in the pathophysiology of ADHD.Acknowledgements
This work was supported by the Natural Science Fund Youth Science Fund Project of China [grant number 82001439], the Medical Scientific Research Foundation of Guangdong Province [grant number A2020327] and the Guangdong Basic and Applied Basic Research Foundation, China (No.2020A1515011436). We would like to thank the participants and their families and the staff at the MRI at the First Affiliated Hospital of Sun Yat-sen University for all their help and support.References
1. Thapar A, Cooper M (2016) Attention deficit hyperactivity disorder. The Lancet 387:1240-12502 Hoogman M, Bralten J, Hibar DP et al (2017)
2. Subcortical brain volume differences in participants with attention deficit hyperactivity disorder in children and adults: a cross-sectional mega-analysis. Lancet Psychiatry 4:310-3193 Lu L, Zhang L, Tang S et al (2019)
3. Characterization of cortical and subcortical abnormalities in drug-naive boys with attention-deficit/hyperactivity disorder. J Affect Disord 250:397-4034 Chen Y, Su S, Dai Y et al (2022)
4. Quantitative susceptibility mapping reveals brain iron deficiency in children with attention-deficit/hyperactivity disorder: a whole-brain analysis. Eur Radiol. 10.1007/s00330-021-08516-25 Tang SL, Zhang GP, Ran QY et al (2022)
5. Quantitative susceptibility mapping shows lower brain iron content in children with attention-deficit hyperactivity disorder. Human Brain Mapping 43:2495-25026 Hagiwara A, Warntjes M, Hori M et al (2017)
6. SyMRI of the Brain: Rapid Quantification of Relaxation Rates and Proton Density, With Synthetic MRI, Automatic Brain Segmentation, and Myelin Measurement. Invest Radiol 52:647-6577 Stuber C, Morawski M, Schafer A et al (2014)
7. Myelin and iron concentration in the human brain: a quantitative study of MRI contrast. Neuroimage 93 Pt 1:95-1068 Rooney WD, Johnson G, Li X et al (2007)
8. Magnetic field and tissue dependencies of human brain longitudinal 1H2O relaxation in vivo. Magn Reson Med 57:308-3189 Wu J, Xiao H, Sun H, Zou L, Zhu LQ (2012)
9. Role of dopamine receptors in ADHD: a systematic meta-analysis. Mol Neurobiol 45:605-62010 Speranza L, di Porzio U, Viggiano D, de Donato A, Volpicelli F (2021)
10. Dopamine: The Neuromodulator of Long-Term Synaptic Plasticity, Reward and Movement Control. Cells 10