5318
Initial experience with Amide Proton Transfer weighted imaging in pediatric neuro-oncology1Department of Radiology and Nuclear Medicine, University Medical Center Utrecht, Utrecht, Netherlands, 2Department of Pediatric Neuro-Oncology, Princess Maxima Center for pediatric oncology, Utrecht, Netherlands, 3BIU MR, Philips Healthcare, Best, Netherlands
Synopsis
Keywords: Neuro, CEST & MT
APTw imaging has shown promising applications in diagnosing adult brain tumors. However, pediatric brain tumors differ from their adult counterparts in terms of clinical, biological, and radiological appearance. Therefore, we investigated if and how APTw imaging can be used in clinical-decision making in pediatric neuro-oncology. First, we showed the repeatability of APTw maps, allowing longitudinal assessment. Then APTw imaging was evaluated in eleven children with a brain tumor. APTw values are higher in pediatric brain tumors compared to normal-appearing white matter. Unlike studies in adults, APTw values were not significant different between low-grade glioma and high-grade glioma in these children.Introduction
Amide proton transfer weighted (APTw) imaging, based on chemical exchange saturation transfer, gives insight into the amount of mobile proteins and peptides in tissue1,2. APTw values could be used as imaging biomarker for tumor activity and tumor aggressiveness since APTw values have shown a correlation with the histopathological cell proliferation marker Ki-673,4. In adult neuro-oncology, APTw imaging shows promising clinical utility in differentiation between low-grade glioma and high-grade glioma and between true tumor progression and pseudoprogression5. Furthermore, APTw imaging could potentially aid in the assessment of treatment effects in adult brain tumors6,7.However, pediatric brain tumors are intrinsically different from their adult counterpart in terms of clinical, biological, and radiological appearance8,9. Consequently, present applications of APTw imaging may not be directly translatable to the pediatric population. Furthermore, to be of use for longitudinal assessment, APTw imaging should be repeatable.
Here we investigated the repeatability of APTw imaging at 3T and evaluated if and how APTw imaging can be used in clinical decision-making in pediatric neuro-oncology.
Methods
AcquisitionA 3T MRI scanner (Ingenia Elition X, Philips Healthcare) was used combined with a 32-channel head receive coil. APTw images were acquired using the APT product developed by Philips Healthcare, i.e. a 3D TSE sequence including seven frequency offsets [Δω=±2.70,±3.50,±4.30 and -1560 ppm]. The frequency offset of 3.5 ppm was acquired three times, with ±ΔTE [-0.4,0,0.4 ms], and was used to create a three-point TSE-Dixon B0-map. Imaging parameters were: saturation time=2s; B1,rms+=2.0 µT; SENSE-factor RL of 1.6; matrix=128x128x10; FOV=230x230x60 mm3; acquired voxel size=1.8x1.8x6.0 mm3; TR/TE=5940/8.3 ms; flip angle=90°.
Processing
Z-spectra were normalized with respect to the frequency offset at -1560 ppm (S0). B0-correction was performed pixel-wise, using the B0-map, shifting, and resampling the z-spectrum accordingly. APTw maps were calculated by MTRasym at 3.5 ppm (Eq.1). If needed (i.e. in presence of cysts which result in high APTw values not reflecting solid tumor tissue5) fluid suppression was incorporated in the MTRasym(Eq.2)10.
$$MTR_{asym}=\frac{S_{sat}[-\Delta\omega]-S_{sat}[\Delta\omega]}{S_0} \tag{1}$$
$$APTw_{fluid suppression}= MTR_{asym}\cdot\left(2-\frac{S_{sat}[\Delta\omega]+S_{sat}[-\Delta\omega]}{S_0}\right)\tag{2}$$
Ssat and S0 represent the frequency offsets at ±3.5 ppm and -1560 ppm, respectively, since Δω is the frequency offset of the molecule of interest.
Repeatability
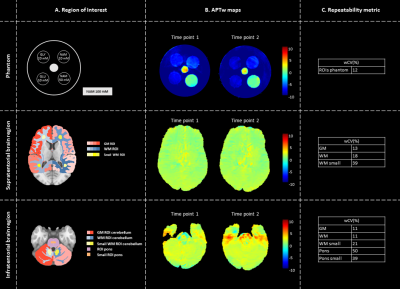
To assess the repeatability, APTw imaging is performed twice with an interval of one week. APTw maps were acquired for a cylindrical phantom (filled with 0.9% saline solution and five submerged falcon tubes at pH ~7.0) and in five healthy volunteers (2m/3f; age:25.8±1.7 years) in supratentorial and infratentorial brain region (Fig.1A).
The repeatability was quantified by calculating the within-coefficient-of-variation (wCV). Regions of interest (ROIs) were defined by manual delineation (phantom) and by applying atlas-based segmentation using the MNI-152 atlas11,12 and Elastix13(healthy volunteers)(Fig.1A).
Clinical applicability
Patient data was evaluated retrospectively. Eleven children with a histopathological and/or radiological confirmed brain tumor were included. Nine patients had a low-grade glioma (pilocytic astrocytoma; location: optic pathway (n=5), posterior fossa (n=3) and basal ganglia (n=1)) and two patients were diagnosed with a pediatric type high-grade glioma. Patient characteristics show a variety of clinical time points and treatments (Tab.1). APTw images were acquired during their regular clinical MRI scan, including FLAIR and T1w-gd scans.
FLAIR and T1w-gd images were registered to the image acquired with a frequency offset of -1560 ppm (rigid registration; Elastix13). Tumor masks were created on the slice of the FLAIR image in which most tumor was present. Furthermore, circular ROIs of 0.2 mL were drawn in normal-appearing white matter (NAWM) of the temporal lobe or parietal lobe. A paired t-test was performed to compare mean APTw values between tumor and NAWM. Differences in mean APTw values between different tumor types were analyzed using one-way ANOVA. Significance threshold was p<0.05.
Results
RepeatabilitySimilar APTw levels were acquired for both time points for all ROIs of the phantom and supratentorial and infratentorial brain region, supported by the wCV (Fig.1). In small WM ROIs the wCV increased by 50% with respect to WM ROIs. In addition, B0-inhomogeneities affect the repeatability, resulting in high wCV, i.e. worse repeatability, in the pontine region. Phantom data indicated that APTw maps are specific for amides.
Clinical applicability
APTw values are higher in tumor compared to NAWM for both low-grade and high-grade types of pediatric brain tumors(p<0.001)(Fig.2 and Fig.3). No significant differences were found in APTw values between low-grade and high-grade glioma(p=0.23). MTRasym with fluid suppression suppressed cystic fluids in pediatric brain tumors (Fig.4).
Discussion
The repeatability of APTw imaging at 3T is investigated, which helps in interpreting the longitudinal assessment of APTw values in brain tumors. In pediatric brain tumors, elevated APTw values are present, comparable to APTw levels reported in adult brain tumors (albeit acquired with different scan protocols)3,4,14. APTw values in brain tumors are on average 2.5-fold higher compared to NAWM, which is greater than the wCV for all brain regions. However, we could not distinguish low-grade and high-grade glioma based on APTw values.Furthermore, high APTw values are present in non-tumorous areas as well. For example, high APTw values near the skull arise most likely from B0-inhomogenities. Therefore, for clinical application, APTw imaging needs a multiparametric approach to focus only on APTw values inside tumors. A cohort study will give further insight into the importance of APTw imaging in pediatric neuro-oncology.
Acknowledgements
We gratefully acknowledge funding of NWO-VIDI 18361-WijnenReferences
[1] van Zijl, P. C. M. & Yadav, N. N. Chemical exchange saturation transfer (CEST): What is in a name and what isn’t? Magn Reson Med 65, 927–948 (2011).
[2] Wu, B. et al. An overview of CEST MRI for non-MR physicists. EJNMMI Physics vol. 3 Preprint at https://doi.org/10.1186/s40658-016-0155-2 (2016).
[3] Su, C. et al. Amide proton transfer imaging allows detection of glioma grades and tumor proliferation: Comparison with Ki-67 expression and proton MR spectroscopy imaging. American Journal of Neuroradiology 38, 1702–1709 (2017).
[4] Togao, O. et al. Amide proton transfer imaging of adult diffuse gliomas: Correlation with histopathological grades. Neuro Oncol 16, 441–448 (2014).
[5] Zhou, J., Heo, H. Y., Knutsson, L., van Zijl, P. C. M. & Jiang, S. APT-weighted MRI: Techniques, current neuro applications, and challenging issues. Journal of Magnetic Resonance Imaging 50, 347–364 (2019).
[6] Sagiyama, K. et al. In vivo chemical exchange saturation transfer imaging allows early detection of a therapeutic response in glioblastoma. Proceedings of the National Academy of Sciences 111, 4542–4547 (2014).
[7] Meissner, J. E. et al. Early response assessment of glioma patients to definitive chemoradiotherapy using chemical exchange saturation transfer imaging at 7 T. Journal of Magnetic Resonance Imaging 50, 1268–1277 (2019).
[8] Merchant, T. E., Pollack, I. F. & Loeffler, J. S. Brain Tumors Across the Age Spectrum: Biology, Therapy, and Late Effects. Seminars in Radiation Oncology vol. 20 58–66 Preprint at https://doi.org/10.1016/j.semradonc.2009.09.005 (2010).
[9] D’Arco, F. et al. Current concepts in radiologic assessment of pediatric brain tumors during treatment, part 1. Pediatr Radiol 48, 1–11 (2018).
[10] Keupp, J. & Togao, O. Magnetization Transfer Ratio based Metric for APTw or CESTw MRI Suppressing Signal from Fluid Compartments - Initial Application to Glioblastoma Assessment. in (ISMRM, 2018).
[11] Fonov, V., Evans, A., McKinstry, R., Almli, C. & Collins, D. Unbiased nonlinear average age-appropriate brain templates from birth to adulthood. Neuroimage 47, S102 (2009).
[12] Fonov, V. et al. Unbiased average age-appropriate atlases for pediatric studies. Neuroimage 54, 313–327 (2011).
[13] Klein, S., Staring, M., Murphy, K., Viergever, M. & Pluim, J. elastix: a toolbox for intensity-based medical image registration. IEEE Trans Med Imaging 29, 196–205 (2010).
[14] Zhou, J. et al. Three-dimensional amide proton transfer MR imaging of gliomas: Initial experience and comparison with gadolinium enhancement. Journal of Magnetic Resonance Imaging 38, 1119–1128 (2013).
Figures