5315
Quantitative relaxometry assessment of brain microstructural abnormality of preschool children with autism spectrum disorder by Synthetic MRI1Department of Radiology, The Third Affiliated Hospital of Zhengzhou University, Zhengzhou, China, 2GE Healthcare, MR Research China, Beijing, China, 3Zhengzhou University People’s Hospital, Zhengzhou, China
Synopsis
Keywords: Neuro, Neuroscience
Quantitative T1/T2 relaxometry can be simultaneously generated from Synthetic MRI (SyMRI) in one single scanning and short time. This study aims to use SyMRI to evaluate the brain structural changes in preschool children with autism spectrum disorder (ASD), and the correlation between T1/T2 and ASD scores, as well as the diagnostic efficacy. We found that Synthetic T1 and T2 could be helpful for brain abnormity assessing, pathophysiological mechanism understanding and diagnosis of ASD.Background or Purpose
ASD is a developmental disorder of the nervous system[1]. Many studies show abnormalities in myelin in the brain of patients with ASD[2-4], and that myelin affects relaxometry. Evaluating the brain of children with ASD from the perspective of relaxometry can help the clinical diagnosis and mechanism research of ASD. SyMRI, as a promising technique, can simultaneously acquire the multiple relaxation mapping and volumetric quantification of the brain in one single scanning[5]. SyMRI has been previously reported for use in central nervous system research, such as detecting myelin in premature infants[6]. In this study, SyMRI was used to assess the relaxation quantification (T1/T2) and brain volume in ASD.Methods
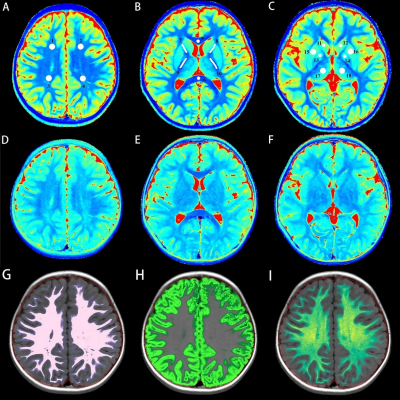
The study included 39 newly diagnosed ASD children (ASD group) and 20 typical developmental children (TD group). Inclusion criteria for the ASD group: (1) 2 ~ 5 years old. (2) Meet the diagnostic criteria for ASD in Diagnostic and Statistical Manual of Mental Disorders-fifth edition (DSM-5)[7]. (3) Childhood Autism Rating Scale (CARS) score equal to or greater than 30. (4) Exclusion of other mental and neurological diseases. (5) No obvious abnormality on conventional MRI. Inclusion criteria for the TD group: (1) 2 ~ 5 years old. (2) No mental or neurological disorders. (3) No obvious abnormality on conventional MRI. Children's developmental quotients were evaluated using the Gesell Developmental Schedules, including adaptive behavior (DQ1), gross motor (DQ2), fine motor (DQ3), language (DQ4), and personal-social behavior (DQ5). A 3.0 T MRI scanner (SIGNA Pioneer, GE Healthcare, Milwaukee, WI) was used to acquire conventional axial T1WI, T2WI, sagittal T1WI and SyMRI (MAGnetic resonance imaging compilation, MAGiC). MAGiC were obtained in axial with TR 4266ms, TE 19.4ms, FOV = 20 cm × 20 cm , matrix = 288 × 224, layer number = 22, layer thickness 4mm, and layer spacing 1mm in total scan time 4min16s. The MAGiC data were then imported into the post-processing software SyMRI (SyntheticMR, Linköping, Sweden, ver. 8.0) to generate brain volume and quantitative maps of T1 and T2 relaxation time for measurement. Regions of interest (ROIs) were manually outlined with ITK-SNAP software (3.6.0) in the center of bilaterally symmetrical brain regions, including (Figure 1): genu of corpus callosum (GCC), splenium of corpus callosum (SCC) and parietal white matter (PWM), frontal white matter (FWM), posterior limb of internal capsule (PLIC), anterior limb of internal capsule (ALIC), thalamus (TH), globus pallidus (GP), head of caudate nucleus (HCN), putamen (PU).Statistical analysis was performed using SPSS software version 26.0. After confirming the numerical data normality, Student t-test or Mann-Whitney U test was applied for group comparison, and Pearson or Spearman correlation analysis was used to evaluate the correlation between relaxometry and clinical scales. The chi-square test was used for comparisons of binary data. The diagnostic efficacy of relaxometry T1/T2 was compared with the receiver operating characteristic (ROC) analysis. P < 0.05 indicated statistically significant.Results
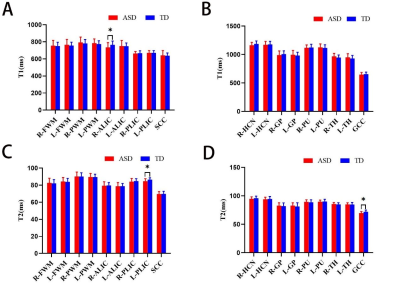
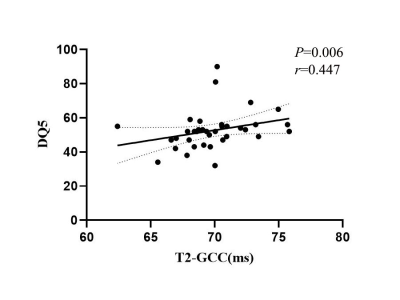
Compared with the TD group, the T1 of the right ALIC, the T2 of both the GCC and the left PLIC in the ASD group were statistically shortened (P < 0.05) (Figure 2). No significant difference in brain volume between two groups. In ASD group, the T2 of the GCC was positively correlated with DQ5 (r =0.447, P = 0.006, Figure 3), but no significant correlation of T1/T2 relaxometry with CARS score. ROC analysis showed that the AUC for T1 in the right ALIC, T2 in the GCC and T2 in the left PLIC was respectively 0.658 (sensitivity: 66.67%, specificity: 70.00%), 0.668(sensitivity: 77.78%, specificity: 60.00%), and 0.697 (sensitivity: 63.89%, specificity: 75.00%).Conclusions
The T1 and T2 relaxometry are associated with myelin maturation and water reduction[8]. Within 6 months after birth, increased content of glycolipids and cholesterol in the myelin sheath was considered to result in a shortened T1, while increased phospholipids and decreased water shortened the T2 in brain tissue[9] as myelin sheath matured. For ASD patient, decreased T1 in ALIC and T2 in GCC and the left PLIC may indicate aggressive development and accumulation of glycolipids, cholesterol and phospholipids, but less water in compared with TD. Thus, there is a more restricted, denser or more viscous microstructural brain tissue environment, such as too dense and restricted nerve fiber bundles, causing the dysfunctional information transmission. Positive correlation of T2 in the GCC with DQ 5 in ASD group indicated that the GCC may be involved in the pathophysiological process of ASD. With moderate AUC, T1 and T2 relaxometry could help the diagnosis of ASD.T1/T2 relaxometry from Synthetic MRI could non-invasively characterize the microstructural abnormalities in the brain of preschool children with ASD, and be helpful in understanding the pathophysiological mechanism and the clinical diagnosis of ASD in children.Acknowledgements
We would like to extend the gratitude and acknowledgements to all study participants.References
[1] Benedetto L, Cucinotta F, Maggio R, et al. One-year follow-up diagnostic stability of autism spectrum disorder diagnosis in a clinical sample of children and toddlers. Brain Sci, 2021; 11(1): 37.
[2] Xiao Z, Qiu T, Ke X, et al. Autism spectrum disorder as early neurodevelopmental disorder: Evidence from the brain imaging abnormalities in 2–3 years old toddlers. J Autism Dev Disord, 2014; 44(7): 1633-1640.
[3] Khanbabaei M, Hughes E, Ellegood J, et al. Precocious myelination in a mouse model of autism. Translational psychiatry, 2019; 9(1): 1-14.
[4] Fu L, Wang Y, Fang H, et al. Longitudinal study of brain asymmetries in autism and developmental delays aged 2–5 years. Neuroscience, 2020; 432: 137-149.
[5] Andica C, Hagiwara A, Hori M, et al. Review of synthetic MRI in pediatric brains: Basic principle of MR quantification, its features, clinical applications, and limitations. J Neuroradiol, 2019; 46(4): 268-275.
[6] Schmidbauer V, Geisl G, Diogo M, et al. SyMRI detects delayed myelination in preterm neonates. Eur Radiol, 2019; 29(12): 7063-7072.
[7] American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. (American Psychiatric Publishing, Washington, DC: Arlington, 2013).
[8] Barkovich A J. Concepts of myelin and myelination in neuroradiology. AJNR Am J Neuroradiol, 2000; 21(6): 1099-1109.
[9] Dubois J, Dehaene-Lambertz G, Kulikova S, et al. The early development of brain white matter: a review of imaging studies in fetuses, newborns and infants. Neuroscience, 2014; 276: 48-71.
Figures