5298
Noninvasive MRI Radiomics Features of adenohypophysis in evaluation of HPG axis activation in children.1Department of Radiology, Tongji Hosptial ofTongji Medical College of Huazhong University of Science and Technology, Wuhan, China, 2Department of Artificial Intelligence,, Julei Technology Company, Wuhan 430000, Hubei, China, Wuhan, China, 3MR Research, GE Healthcare, Beijing 100176, China, Beijing, China
Synopsis
Keywords: Adolescents, Pediatric, hypothalamic-pituitary-gonadal axis
we report for a low-cost and rapid radiomics model based on MRI data in evaluation of hypothalamic-pituitary-gonadal (HPG) axis activation in children. In our study, radiomics model base on CUBE T1WI showed good prediction of HPG axis activation with the AUC of 0.84 in the training set and 0.81 in the test set. The AUC of the radiomics model was higher than that of aPV and aPH in the training set. In results of DCA analysis, radiomics signature showed higher net benefit than aPV and aPH models.The MRI radiomics model may serve as a noninvasive predictor of HPG axis activation.Background or Purpose
The hypothalamic-pituitary-gonadal (HPG) axis is active in the embryonic and at the early postnatal stages of human life, and is subsequently restrained during childhood. Its reactivation culminates in puberty initiation period [1]. The status of HPG axis is important in evaluation of physiological and pathological puberty onset before the ages of 12 and 14 respectively for girls and boys [2].The gonadotropin-releasing hormone (GnRH) stimulation test is regarded as the gold standard to assess activation of HPG axis and diagnose physiological and pathological puberty onset, PP, CPP, Constitutional delay in growth and puberty (CDGP) and congenital hypogonadotropic hypogonadism (cHH)[3]. However, GnRH stimulation testing is necessary, but not easy to implement because of required hospital admission, multiple sampling in addition to cost and availability. Moreover, frequent blood collection can impose psychological, financial, and time burdens on children’s patients.
The objective of this study was to develop and validate a noninvasive, low-cost and rapid radiomics model based on MRI CUBE T1 image in evaluation of HPG axis activation in children.
methods
A total of 239 children who received a hypophysial MRI scan and GnRH stimulation testing from April 2021 to September 2021 were enrolled in our study and divided into the training and test sets using stratified random sampling method. Radiomics features were generated using the least absolute shrinkage and selection operator (LASSO) with 10-fold cross-validation. The performance of radiomics model was evaluated using receiver operating characteristic (ROC) and decision curve analysis (DCA).Results
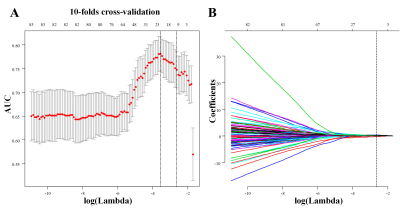
A total of 239 cases were identified (83 boys and 156 girls; mean age, 8.70 ± 0.15 years). This cohort was randomized at a seven-to-three ratio into the training and test cohorts. There were 168 cases (65 boys and 103 girls; mean age, 8.78 ± 0.19) in the training cohort and 71 cases (18 boys and 53 girls; mean age, 8.51 ± 0.25) in the test cohort.Of Radiomics features, 851 features were reduced to 10 best potential predictors in the training cohort (10:1 ratio), and used with nonzero coefficients in the LASSO logistic regression model. These features were presented in the Radiomics signature.There was a significant difference of Radiomics Signature between LH ≥ 5 IU/L (LH+) and LH-<5 IU/L (LH-) patients in the training cohort (P <0 .001) and in the test cohort (P < 0.001). LH+ patients generally had higher radiomics signature in the train set. A logistic regression analysis identified 10 radiomic signatures using LH level as an independent predictor. The model that incorporated the above independent predictors was developed and presented as the Radiomics Signature( Figure 1).
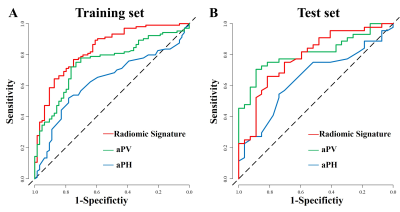
The sensitivity, specificity and AUC of the CUBE T1WI model for predicting HPG axis activation were 76.92% (49 of 64 patients), 75.00% (78 of 104 patients ) and 0.84 (95% CI: 0.78, 0.90) in the training set and 77.27% (21 of 27 patients, 95% CI: 63.64, 88.64), 74.07% (34 of 44 patients) and 0.81 in the test set The radiomics model showed higher AUC than aPV and aPH in training set (0.84 vs 0.75 [P =0.001] and 0.84 vs 0.63 [P<0.001], respectively). There was no difference between the AUC of the radiomics model and aPV in test set (0.81 vs 0.78, P =0.58), but higher than that of aPH (0.81 vs 0.65, P =0.03)( Figure 2) .
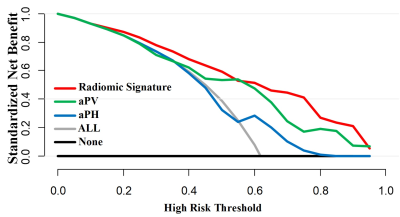
For DCA analysis, Radiomics Signature model showed higher net benefit than aPV and aPH models. When the threshold exceeded 0.80, aPH reversely showed the net benefit at zero while Radiomics Signature and aPV still had a high net benefit( Figure 3).
Discussions and Conclusions
HPG axis dysfunction causes symptoms such as precocious puberty, delayed puberty, and tumor formation [4].Due to the disadvantages of GnRH stimulation test,it is important to simplify the GnRH stimulation test or seek alternative methods to more easily evaluate the HPG axis activation [5].A straight-forward non-invasive and radiographic diagnosis model for HPG axis activation in pre- and at- puberty children was built in our study. The radiomics model successfully stratified patients with the standard reference of the peak LH. The three-dimensional CUBE T1WI radiomics model was constructed and validated in a large-scale cohort from a single institution and yielded good diagnostic performance on assessment of HPG axis activation with AUC = 0.81, and the diagnostic value of the radiomics model we built was better than single-factor (aPV-only and aPH-only) models.
In conclusions, the MRI-based radiomics model showed good classification performance on diagnosis of HPG axis activation in pre- and at- puberty children. It could serve as a noninvasive predictor of HPG axis activation, potentially providing valuable information in the diagnosis of patients with PP, CPP, CDGP and cHH.
Acknowledgements
We thank for Weiyin Vivian Liu (GE Healthcare, Beijing) providing support.References
1.Spaziani M, Tarantino C, Tahani N, Gianfrilli D, Sbardella E, Lenzi A, Radicioni AF. Hypothalamo-Pituitary axis and puberty. Mol Cell Endocrinol. 2021 Jan 15;520:111094. doi: 10.1016/j.mce.2020.111094. Epub 2020 Dec 1.
2. Carel JC, Eugster EA, Rogol A, Ghizzoni L, Palmert MR; ESPE-LWPES GnRH Analogs Consensus Conference Group, Antoniazzi F, Berenbaum S, Bourguignon JP, Chrousos GP, Coste J, Deal S, de Vries L, Foster C, Heger S, Holland J, Jahnukainen K, Juul A, Kaplowitz P, Lahlou N, Lee MM, Lee P, Merke DP, Neely EK, Oostdijk W, Phillip M, Rosenfield RL, Shulman D, Styne D, Tauber M, Wit JM. Consensus statement on the use of gonadotropin-releasing hormone analogs in children. Pediatrics. 2009 Apr;123(4):e752-62.
3. Howard SR, Dunkel L. Delayed Puberty-Phenotypic Diversity, Molecular Genetic Mechanisms, and Recent Discoveries. Endocr Rev. 2019 Oct 1;40(5):1285-1317. doi: 10.1210/er.2018-00248. Erratum in: Endocr Rev. 2020 Feb 1;41(1):
4. Park YW, Kang Y, Ahn SS, Ku CR, Kim EH, Kim SH, Lee EJ, Kim SH, Lee SK. Radiomics model predicts granulation pattern in growth hormone-secreting pituitary adenomas. Pituitary. 2020 Dec;23(6):691-700. doi: 10.1007/s11102-020-01077-5. PMID: 32851505.
5. Yeh SN, Ting WH, Huang CY, Huang SK, Lee YC, Chua WK, Lin CH, Cheng BW, Lee YJ. Diagnostic evaluation of central precocious puberty in girls. Pediatr Neonatol. 2021 Mar;62(2):187-194.
Figures