5296
Structural and Functional Brain Alterations in Children with Autism Spectrum Disorder1Department of Radiology, Shanghai Tenth People’s Hospital, Tongji University School of Medicine, Shanghai, China, 2Philips Healthcare, Shanghai, China
Synopsis
Keywords: Neuro, fMRI (resting state)
MRI is an important tool available to detect brain functional and structural abnormalities of autism spectrum disorder (ASD) patients. In this study, we found that children with ASD have increased changes in structure and both increased and decreased changes in function in the brain, which suggests that there may be some underlying pathogenic mechanism in the brain of ASD. With the combination of brain structural and functional analysis can provide a new imaging perspective for understanding the neural mechanism of ASD.Introduction
Autism spectrum disorder (ASD) has been widely recognized as a complex neurodevelopmental disorder characterized by impaired social interaction and repetitive behaviors. Previous neuroimaging studies suggest that ASD patients have structural and functional abnormalities in the brain.1,2 The brain structure-function interaction is rather complex, suggesting that function may be constrained or compensated by structure while structure can be modified by the function. Voxel-based morphometry (VBM) is a technique for automatic and objective segmentation of brain structure using MR imaging based on the voxel level. Amplitude of low-frequency fluctuation (ALFF) , regional homogeneity (ReHo) and degree centrality (DC) based on resting-state fMRI are useful data-driven methods to characterize brain function and activity.3 The aim of the current study is to investigate structural and functional alterations in the brain in children (3–13 years old) with ASD compared with typically developing controls (TDs).Methods
One hundred and forty-four children with ASD were tested by pediatricians and enrolled in this study from June 2021 to November 2022. Forty-seven TDs were recruited from the community. Anatomical T1 weighted images and resting-state fMRI images were acquired on an Ingenia CX 3.0-T MRI scanner (Philips Healthcare, Best, the Netherlands) with a 32-channel head coil. All children were examined under sedation using chloral hydrate and one parent and one doctor for each child were present throughout the duration of the scan. To preprocess structural images, the SPM8 and VBM8 toolboxes were applied and performed in MATLAB R2013b (Mathworks, Natick, MA). VBM can be used to analyze brain structure based on voxel level and segment images. Grey matter images were spatially normalized to the MNI standard space and a Gaussian kernel of 8 mm FWHM was used to smooth. Using two-sample t-tests to analyze the gray matter volume (GMV) changes in the brain. The preprocessing of resting-state fMRI data was performed in MATLAB R2013b (Mathworks, Natick, MA) with RESTplus software.4 The first ten time points of fMRI data were removed. Because of using multiband scanning, slice timing was not performed.5 After realigning, six children with ASD and five TDs had excessive head motion (≥±3 mm in any axis). Then the processed images were then normalized by DARTEL toolbox using T1 image new segment. A Gaussian filter with 6mm FWHM was used to smooth the data (for ReHo and DC, this step was performed during ReHo and DC calculation). The Friston 24 motion parameters, white matter, and cerebrospinal fluid signals were regressed as covariates. Finally, band-pass filtering (0.01–0.08 Hz) was performed to remove the effects of high-frequency noise (for ALFF, the ALFF calculation replaced this step). To explore the functional alterations between the two groups, two-sample t-tests were performed on the ALFF, ReHo and DC maps.Results
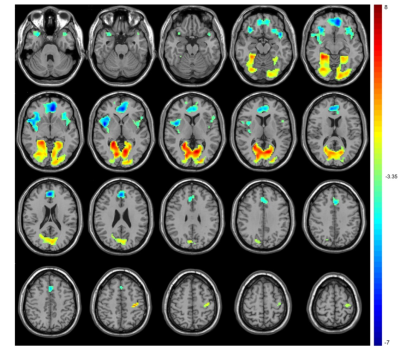
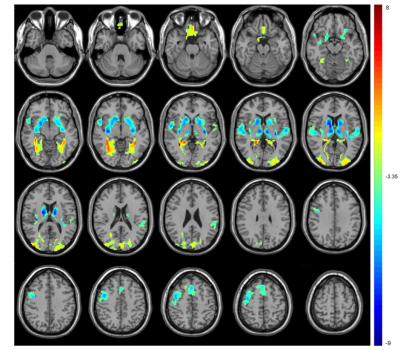
Compared with TDs, VBM analyses revealed increased GMV in the gyrus rectus, left inferior parietal gyrus and right superior parietal gyrus in ASD children (P < 0.001, cluster size > 100) (Fig 1). ASD children showed lower ALFF in the bilateral medial superior frontal gyrus and higher ALFF in the bilateral calcarine fissure and precentral gyrus. The ReHo decreased in the left superior temporal gyrus, right insula and bilateral anterior cingulate gyrus and increased in the bilateral calcarine fissure and left postcentral gyrus. The DC values decreased in the right caudate nucleus, bilateral superior temporal gyrus, right precentral gyrus and bilateral supplementary motor area, while increased in the bilateral gyrus rectus, fusiform gyrus, and middle occipital gyrus. (voxel-wise P < 0.001, FDR-corrected cluster-wise P < 0.05) (Fig 2-4). The results of including the participants with excessive head motion remained similar to the results of excluding these participants.Discussion
In this study, increased GMV has been found in the gyrus rectus and parietal lobes. The gyrus rectus could play a role in leading to more difficulties in social cognition6 and the parietal lobes are responsible for language and vision. This study found spontaneous activity changes in multiple brain regions such as temporal and occipital lobes which are responsible for visual and cognitive abilities, which could help to further understand the clinical characteristics of children with ASD. ALFF and ReHo are two important indicators demonstrating neural intensity and neural coherence. In this study, ALFF and ReHo were both increased in the calcarine fissure which was an important part of the primary visual cortex, becoming the mechanism of abnormal visual processing in ASD. DC is an index of the total weights of connections for a brain region. Caudate is involved in controlling and regulating movement and decreased DC may cause repetitive motor behaviors. In this study, the gyrus rectus was overlapped between functional and structural changes, suggesting the importance of the region in the mechanism of ASD. Previous studies found alterations of brain structural and functional asymmetry in ASD.7,8 In this study, several brain regions included bilateral sides and the alterations were different between the left side and right side. We also found within or without participants with excessive head motion, the results were similar.Conclusion
Neuroimaging analysis of brain structural and functional changes can provide a new imaging perspective for understanding the neural mechanism and providing biomarkers for the clinical diagnosis of ASD.Acknowledgements
We would like to thank Shanghai Tenth People’s Hospital and Philips Healthcare for supporting this research. We also thank all participants and their families for their cooperation in this study.References
1. Sato W, Uono S. The atypical social brain network in autism: advances in structural and functional MRI studies. Curr Opin Neurol, 2019, 32: 617-621.
2. Li X, Zhang K, He X, Zhou J, Jin C, Shen L, et al. Structural, Functional, and Molecular Imaging of Autism Spectrum Disorder. Neurosci Bull, 2021, 37: 1051-1071.
3. Lv H, Wang Z, Tong E, Williams L M, Zaharchuk G, Zeineh M, et al. Resting-State Functional MRI: Everything That Nonexperts Have Always Wanted to Know. American Journal of Neuroradiology, 2018, 39: 1390-1399.
4. Jia X, Wang J, Sun H, Zhang H, Liao W, Wang Z, et al. RESTplus: an improved toolkit for resting-state functional magnetic resonance imaging data processing. Science Bulletin, 2019, 64: 953-954.
5. Smitha K A, Arun K M, Rajesh P G, Joel S E, Venkatesan R, Thomas B, et al. Multiband fMRI as a plausible, time-saving technique for resting-state data acquisition: Study on functional connectivity mapping using graph theoretical measures. Magn Reson Imaging, 2018, 53: 1-6.
6. Li W, Lou W, Zhang W, Tong RK, Jin R, Peng W. Gyrus rectus asymmetry predicts trait alexithymia, cognitive empathy, and social function in neurotypical adults. Cereb Cortex. 2022 May 15:bhac184.
7. Postema M C, van Rooij D, Anagnostou E, Arango C, Auzias G, Behrmann M, et al. Altered structural brain asymmetry in autism spectrum disorder in a study of 54 datasets. Nat Commun, 2019, 10: 4958.
8. Cardinale R C, Shih P, Fishman I, Ford L M, Muller R A. Pervasive rightward asymmetry shifts of functional networks in autism spectrum disorder. JAMA Psychiatry, 2013, 70: 975-82.
Figures

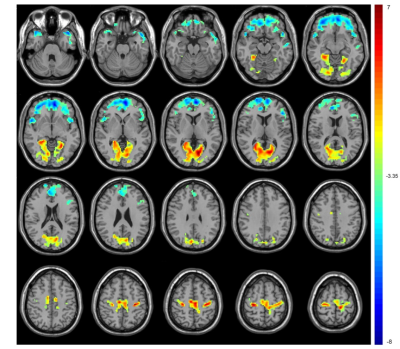
Figure 2 The significant alterations of ALFF between the ASD and TD groups. ASD children showed lower ALFF in the bilateral medial superior frontal gyrus and higher ALFF in the bilateral calcarine fissure and precentral gyrus. Warm color indicates that ALFF is higher in the ASD children than in the TDs, and vice versa.