5289
Heterogeneous regional growth process and speed in marmoset brain: multi-modal longitudinal MRI study
Akiko Uematsu1, Makoto Fukushima1, Junich Hata2, Ayako Murayama1, Noriyuki Kishi3, Takuya Hayashi1, and Hideyuki Okano3,4
1RIKEN, Hyogo, Japan, 2Tokyo Metropolitan University, Tokyo, Japan, 3RIKEN, Saitama, Japan, 4Keio University School of Medicine, Tokyo, Japan
1RIKEN, Hyogo, Japan, 2Tokyo Metropolitan University, Tokyo, Japan, 3RIKEN, Saitama, Japan, 4Keio University School of Medicine, Tokyo, Japan
Synopsis
Keywords: Normal development, Animals
“The soul of a child of three is the same at 100” may be also true among all the primates. Here, we delineate the robust value changes of T1w, T2w, and DTI metrics for the first 2 to 3 months after birth in common marmoset brain. Different temporal changes across brain regions were found by Fixel-based analysis, suggesting that different neuronal contributors of age-related structural changes. Integrating multi-modal MRI images provided more detailed insight of tissue property changes especially through development as compared with a single-modal image analysis.Introduction:
Proper brain development at early life stage is crucial for well-being and cognitive function at later life stages. However, the detailed biological process of early postnatal brain development has been largely unknown in primates. Common marmoset (Callithrix jacchus) is a non-human primate (NHP), living in extended families like human beings. Its relatively fast growth and high fertility have the benefit of studying primate development. Here, using a cutting-edge MRI technique, we investigated developmental profiles of brain at early life stage with longitudinal multi-modal images.Methods:
The animals are normally developing marmosets (N=21, 14 males, age range: 2-26 weeks old). A total of 86 time points of structural brain data (1 to 10 times per animals) were acquired with a 9.4T animal MRI scanner (Biospec 94/30, Bruker BioSpin: Germany). All experimental procedures were approved by the Experimental Animal Committee of RIKEN (Approval number H29-2-244). The structural data included multi-modal imaging with T1 weighted (T1w), T2 weighted (T2w), and diffusion-weighted MRI (dMRI) data to examine gray and white matter structure and microstructure, acquired with the following sequences: T1w with 3D MDFE (TR/TE = 6000 ms/2.0 ms, resolution= 0.27x0.27x0.54 mm3), T2w with RARE (TR/TE = 4000 ms/11.0 ms, resolution=0.27x0.27x0.54 mm3), dMRI with a multi-shot (four shots) EPI (TR/TE = 3000 ms/25.57 ms, resolution=0.35x0.35x0.70, 30 diffusion directions at b = 1000 s/mm2 with two b = 0 s/mm2 and two opposing phase encoding b = 0 s/mm2).For data processing, concerning recent practice of data sharing in human research field, our current dataset is organized in BIDS format and processed by a NHP version of human connectome project (HCP) pipeline1, customized for developing marmoset data collected by Bruker MRI scanne (Figure 1). The preprocess of dMRI was also basically followed HCP pipeline, but we also used MRtrix3-provide Fixel-based analysis2 for addressing age-related changes. We also performed regression analysis using a statistical software R (ver 4.03).
Results:
Multi-modal data delineated the typical development of marmoset brain during their half year of life, relative to the preschool period in human. Namely, a rapid volumetric increase occurred during first 3 months after birth, and its growth speed gradually got slow down. Such pattern was also observed in mean FA in whole white matter. The intracranial volume in marmoset increased nonlinearly from 2 weeks (6392 ± 788 mm3) to 6 months (9503 ± 624 mm3), whereas body weight was linearly increased from 45.8 ± 4.5 g to 278.0 ± 53.9 g.The developmental pattern of temporal profile was not spatially uniform across brain regions. For example, the low T1w signal in anterior commissure at under 2 months old, whereas the high T2w in internal capsule and corona radiata at under 1 month old, all of which converted into the high/low signal at later ages (Figure 2). The GAM regression models of cerebral gray and white matter volumetric development and the DTI metrics in anterior commissure, corpus callosum, and internal capsule regions also confirmed this heterogenic growth speed (Figure 3). In addition, our results of Fixel-based analysis also suggested that the neuronal process along with development should be different among regions: Although significant age effect on fiber bundles were observed in the whole brain, the development of frontal white matter would more likely be due to the increase of fiber density, whereas those of anterior commissure and cerebellum would be due to the increase of fiber bundle diameters.
There were no significant lateral and sexual differences in marmoset brain development.
Discussion:
Here, benefitting from its early maturity and prolificacy, we used a non-human primate, common marmoset to delineate a typical developmental pattern of the brain structure by collecting longitudinal MRI data. Our data shows that especially the period between 2 weeks and 2 months old was the most evident in growth rate, but the growth patterns and length were heterogenous across brain regions. Such exponential developmental pattern is consistent among primates including humans. However, unlike human beings there were no sexual or lateral difference in marmoset probably because of similar head and body size between females and males and no hand preferences.The changes of T1w and T2w signals through development suggested the postnatal white matter maturational process begins from internal capsule. Internal capsule contains both motor and sensory-related fibers, connecting between subcortical and cortical regions through thalamus. The value of mean FA in internal capsule also rapidly increased in first 8 weeks after birth and got stabilized. The infant marmoset receives external stimuli like parent’s licking and keeps clinging to their caregivers until 4 weeks and then starts moving around by itself. These findings suggest that the regions related to basic sensory and motor function need to mature fast, rapidly changing neuronal structures (the enlargement of neuronal soma, myelinogenesis, dendric arborization, synaptogenesis, etc.) to adapt to their external environment, possibly resulting in the volumetric and diffusion changes along with age.
Acknowledgements
This research was supported by Agency for Medical Research and development (AMED) [grant number: JP15dm0207001 and JP18dm0307006References
- T. Hayashi, et al. "The nonhuman primate neuroimaging and neuroanatomy project." NeuroImage 229 (2021): 117726.
- D.A.Raffelt, et al. "Connectivity-based fixel enhancement: Whole-brain statistical analysis of diffusion MRI measures in the presence of crossing fibres." Neuroimage 117 (2015): 40-55.
Figures
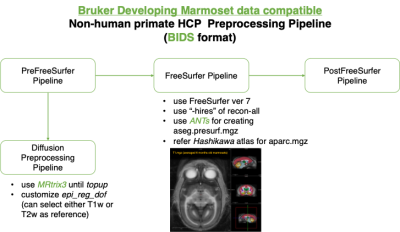
Bruker marmoset data compatible NHP pipeline.
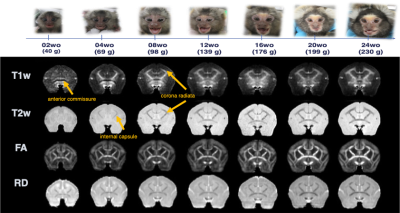
Age-related image contrast changes of T1w, T2w, FA, and RD.
The images were averaged by each age. the marmoset in the pictures is the same individual.
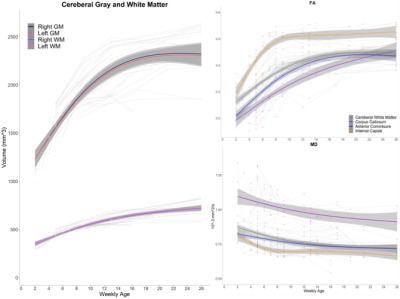
Typical developmental trajectories of marmoset brain structures from 2 weeks to 6 months old.
Each individual trajectory is drawn with spaghetti plots. Group regression lines were estimated by gam.
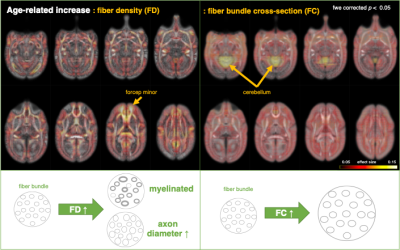
Fixel analysis to examine weekly age effect.
Individual effect was regressed out
DOI: https://doi.org/10.58530/2023/5289