5285
In Vivo Imaging of LC-NE Integrity: Mechanisms Underlying Heath Disparity for Alzheimer’s Disease1NYUSoM, New York, NY, United States
Synopsis
Keywords: Alzheimer's Disease, PET/MR
Although blacks are at 2-3 times higher prevalence rate of developing AD, blacks have been under-included in many prominent AD clinical trials. The current biomarker classification system (ATN) can’t explain the increased prevalence in blacks of both AD and vascular risk factors for AD such as diabetes and hypertension when compared to whites. Our decade-long PET/MR studies have demonstrated a special vulnerability of locus coeruleus (LC) to aging and stress. Our recent study showed that a faster decline of LC function occurs in blacks. Thus, imaging LC represents a novel biomarker approach to mechanisms underlying health disparity for AD.Introduction
Despite studies suggesting that blacks may be at greater risk of developing Alzheimer’s Disease (AD), with 2-3 times higher prevalence rate of cognitive impairment than whites, there have been few studies investigating racial disparities, and blacks have been under-included in many prominent U.S. AD biomarker and clinical trials 1. The current biomarker classification system (i.e., the ATN model)2 may not fully account for racial disparity and can’t explain the increased prevalence in blacks of both AD and vascular risk factors for AD such as diabetes and hypertension when compared to whites. Research on cognitive aging has traditionally focused on how decline in various cortical and hippocampal regions influence cognition. However, tau pathology emerges decades before amyloid pathology, appearing first in the brainstem; particularly in the locus coeruleus (LC), the source of brain’s norepinephrine (NE). Further, postmortem studies suggest that loss of LC neurons better predicts severity of AD symptoms than Aβ/neurofibrillary tangle pathology in any other brain region 3. Our decade-long studies in humans using a norepinephrine transporter (NET)-selective radiotracer ([11C]MRB) have demonstrated a special vulnerability of LC to aging and stress (Figure 1) 4, 5, 6 (see Review7, 8 and references cited within). In this study, we investigated the potential ethnicity effects on NET availability to provide molecular insight into the mechanisms underlying health disparity and increased prevalence in AA for preclinical AD.Methods
Healthy adult black/African American (AA) and white participants with no current medical problems and no psychiatric history were recruited. NET-selective radiotracer ([11C]MRB) with the same production and imaging protocols (Dr. Ding’s IND) was used for all studies examined. The source of the classification for race/ethnicity was based on self-identification. In this report, data analysis on healthy adults (AA, N=14; 7 M, ages 23-49; avg. 33.8 ± 7.2) and non-Hispanic-whites (nhW, white) (N = 16; 11M, ages 24-55; avg. 39.5 ± 10.7) were examined and compared. Co-registration of PET (dynamic [11C]MRB), MRI and the FreeSurfer (FS) atlas images of each individual was performed to generate regional time-activity curves (TAC) using Firevoxel (https://wp.nyu.edu/Firevoxel). Binding potential (BPND) were determined using MRTM2 (t2* 20 min and k2’ 0.021 min-1 with occipital as the reference region). Annual percent change (APC) of BPND was calculated based on linear regression (APC = 100 × (em – 1), m: slope)9 and effects of age, gender and ethnicity on tracer binding were evaluated.Results
For all HC (N=30), with both genders and all races included, age-sensitive decline on MRB-NET binding (index of NET availability) was observed; e.g., 0.3-0.5%/yr decline for hippocampus, brain stem and olfactory. Interestingly, out of all investigated brain ROIs (16), mean BPND values (NET availability) of each ROI from AA were consistently higher, as compared with those of nhW (p < 3.0 e-10). On the contrary, the annual decline rate (APC) of regional NET availability was consistently much higher for AA than for white (p < 0.00001) (Figures 2 & 3). Further, the decline rates of regional brain ROIs were particularly faster in AA males: e.g., 2-3%/yr vs. 0.14-0.23%/yr in thalamus and brain stem for AA males vs. white males (p < 0.00001).Conclusions
Our study demonstrates that the decline of the LC-NE system observed in normal aging, measured via PET imaging of NET availability, may be a potential early biomarker for preclinical AD in midlife ─ a critical time of biological and psychosocial transition ─ thus creating a “window of opportunity” for targeting prevention and treatment. Our study also sheds light upon fundamental yet elusive questions on the underlying mechanisms for the racial/ethnic disparity in preclinical AD stages. Our data in AA and participants reveals, for the first time, that a much faster decline of LC-NE function occurs in blacks, starting in the mid-30s and particularly in AA males, possibly caused by cumulative stress to socioeconomic disadvantage and racial discrimination, and may be responsible for the different disease expression among blacks. That is, the LC neurons are in a strategic position to exert a broad influence over CNS function, particularly in the integration and orchestration of the adaptive CNS response to various stressors or challenges (Figure 1)10,11. Thus, NET availability imaging represents a novel biomarker approach to racial-dependent strategies for diagnosis and assessment of therapeutic interventions.Acknowledgements
Supported for this study provided by National Institute (Grant Nos. DA019062, R56DA19062, 1P41EB017183-01A1, 1R01AG072644-01, 5R21AG064474-02, 3R21AG064474-02S, 1R21AG067549-01).References
1. Shin, J.; Doraiswamy, P. M., Underrepresentation of African-Americans in Alzheimer's Trials: A Call for Affirmative Action. Front Aging Neurosci 2016, 8, 123.
2. Jack, C. R., Jr.; Bennett, D. A.; Blennow, K.; Carrillo, M. C.; Dunn, B.; Haeberlein, S. B.; Holtzman, D. M.; Jagust, W.; Jessen, F.; Karlawish, J.; Liu, E.; Molinuevo, J. L.; Montine, T.; Phelps, C.; Rankin, K. P.; Rowe, C. C.; Scheltens, P.; Siemers, E.; Snyder, H. M.; Sperling, R., NIA-AA Research Framework: Toward a biological definition of Alzheimer's disease. Alzheimer's & dementia : the journal of the Alzheimer's Association 2018, 14 (4), 535-562.
3. Mather, M.; Harley, C. W., The Locus Coeruleus: Essential for Maintaining Cognitive Function and the Aging Brain. Trends Cogn Sci 2016, 20 (3), 214-226.
4. Ding, Y. S.; Lin, K. S.; Garza, V.; Carter, P.; Alexoff, D.; Logan, J.; Shea, C.; Xu, Y.; King, P., Evaluation of a new norepinephrine transporter PET ligand in baboons, both in brain and peripheral organs. Synapse 2003, 50 (4), 345-52.
5. Ding, Y. S.; Singhal, T.; Planeta-Wilson, B.; Gallezot, J. D.; Nabulsi, N.; Labaree, D.; Ropchan, J.; Henry, S.; Williams, W.; Carson, R. E.; Neumeister, A.; Malison, R. T., PET imaging of the effects of age and cocaine on the norepinephrine transporter in the human brain using (S,S)-[(11)C]O-methylreboxetine and HRRT. Synapse 2010, 64(1), 30-8.
6. Pietrzak, R. H.; Gallezot, J. D.; Ding, Y. S.; Henry, S.; Potenza, M. N.; Southwick, S. M.; Krystal, J. H.; Carson, R. E.; Neumeister, A., Association of posttraumatic stress disorder with reduced in vivo norepinephrine transporter availability in the locus coeruleus. JAMA Psychiatry 2013, 70 (11), 1199-205.
7. Ding, Y. S., Progress in PET imaging of the norepinephrine transporter system. In PET and SPECT of Neurobiological Systems, al., R.A.J.O.D. e. al.: Springer-Verlag Berlin Heidelberg: 2014; pp 561-584.
8. Ding, Y. S.; Lin, K. S.; Logan, J., PET imaging of norepinephrine transporters. Curr Pharm Des 2006, 12 (30), 3831-45.
9. Clegg, L. X.; Hankey, B. F.; Tiwari, R.; Feuer, E. J.; Edwards, B. K., Estimating average annual per cent change in trend analysis. Stat Med 2009, 28 (29), 3670-82.
10. Chrousos, G. P., Stress and disorders of the stress system. Nat Rev Endocrinol 2009, 5 (7), 374-81.
11. Chrousos, G. P.; Gold, P. W., The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. JAMA 1992, 267 (9), 1244-52.
Figures
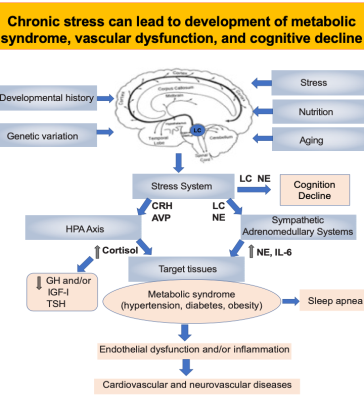
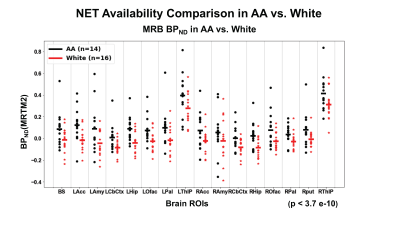
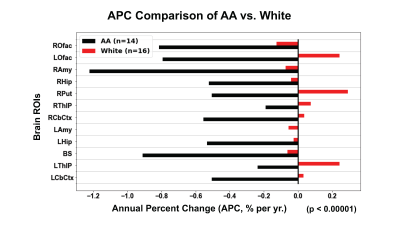
Figure 3. Annual percent change (APC) comparison of AA vs. white participants
ROfac: right olfactory; LOfac: left olfactory; RAmy: right amygdala; RHip: right hippocampus; RPut: right putamen; RThlp: right thalamus proper; RCbCtx: right cerebral cortex; Lamy: left amygdala; LHip: left hippocampus; BS: brainstem; LThlp: left thalamus proper; LCbCtx: left cerebral cortex