5270
Neuroimaging Correlates of Functional Motor Changes in Cognitively Impaired Cohorts1Barrow Neurological Institute, Phoenix, AZ, United States, 2Arizona State University, Tempe, AZ, United States
Synopsis
Keywords: Data Analysis, Diffusion/other diffusion imaging techniques
The objective of this study was to assess white matter microstructural differences between groups of healthy controls (HC), subjective memory complaints (SMC), and cognitive impairment (CI) through multi-shell free-water (FW) corrected DTI (FW-DTI) metrics and by the peak width of skeletonized mean diffusivity (PSMD). Additionally, brain correlations at the voxel level between a functional motor measure (two-bean transfer) and DTI metrics/PSMD were explored.Introduction
Mild cognitive impairment (MCI) is an intermediate state between normal aging and dementia1. Individuals with MCI show greater cognitive impairment than expected based on age, but do not meet commonly accepted criteria for dementia2. However, MCI is thought to represent a high-risk for developing dementia, in particular for Alzheimer’s disease (AD)3. Subjective memory complaints (SMC) are also common in older adults, and these may be related to neuropsychiatric disorders such as anxiety and depression4. Additionally, people with SMC are often aware of a perceived decline in cognitive abilities despite the absence of deficits on clinical assessments5.Few studies have assessed the underlying neuropathological changes associated with SMC and MCI, which is critical for clinical management of these disorders. Magnetic resonance imaging (MRI)-based biomarkers with diffusion tensor imaging (DTI) are sensitive to white matter (WM) microstructure and may be useful to study these changes in cognition and dementia6.
In this preliminary study, we analyzed WM microstructural differences between groups of healthy controls (HC), SMC, and cognitive impairment (AD+MCI subjects; CI). WM microstructure differences were assessed by multi-shell free-water (FW) corrected DTI (FW-DTI)7 metrics and by the peak width of skeletonized mean diffusivity (PSMD)8. As new research shows that motor function may be impaired in preclinical AD, this study explored voxel-based correlations between a functional motor measure (two-bean transfer)9 and DTI metrics.
Methods
This study included 7 HC (mean age (standard deviation (S.D.)): 72.7 (8.0) years), 8 SMC (mean age (S.D.): 67.8 (4.9) years), and 9 CI (mean age (S.D.): 74.1 (5.8) years). A standard neuropsychological examination was conducted for SMC and CI cohorts to confirm participants’ cognitive status. Subjects performed a motor task that involved using a spoon to transfer two raw kidney beans at a time from one cup to another with both dominant and nondominant hands. Performance on this task was quantified as the intrasubject standard deviation (nISD) in trial time across four trials.Diffusion MRI was performed at 3T (Philips) using a multi-shell acquisition with 60 diffusion directions (b=500, 1000, and 2500 s/mm2) and one B0 image. DTI pre-processing was performed by MRtrix310, FSL11, and the Advanced Normalization Tools (ANTs)12. Multi-shell FW metrics were computed by the Diffusion Imaging in Python (Dipy)13, yielding FW-corrected fractional anisotropy (FW-FA) and FW index.
To study differences across groups, the one-way ANCOVA test was performed with R and R-Studio, by a linear model function, using age and sex as covariates. Post-hoc comparisons were analyzed using Tukey's test. For all statistical analysis, p-values were corrected by FDR and Bonferroni (only for post-hoc). 3dClustSim (AFNI)14 was used to estimate the probability of false positive (noise-only) clusters. Effect-sizes (eta-squared (η2) for ANCOVA and Hedge’s g for post-hoc, respectively) were also calculated by R. PSMD was computed using FSL and an in-house script. Voxel-based correlations with the nISD were assessed using a linear model in R for FW-DTI metrics and by the Spearmen’s correlations coefficient for PSMD.
Results
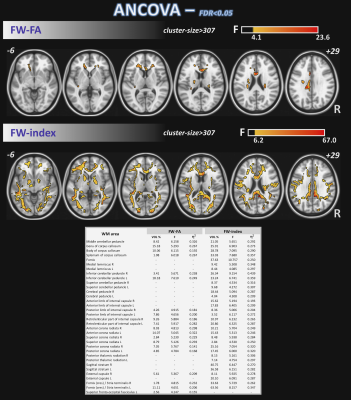
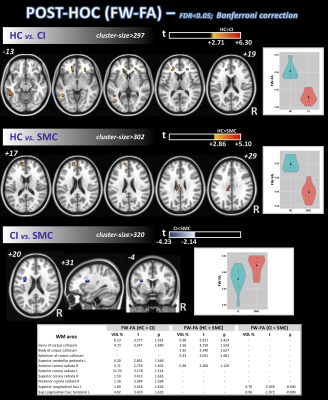
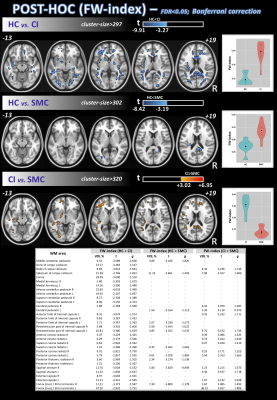
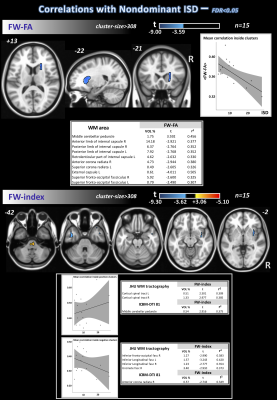
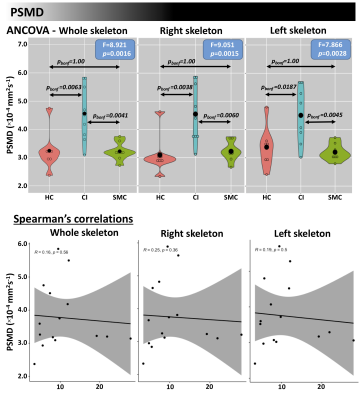
Differences in nISD across groups were found (Kruskal-Wallis: =6.28; p=0.043). ANCOVA identified differences across groups (FDR<0.05) for FW-FA and FW index, mainly in corpus callosum, anterior corona radiata, and fornix (Figure 1). Post-hoc analyses (FDR<0.05+Bonferroni) showed higher FW-FA in HC than SMC and CI. Additionally, compared with CI group, SMC showed higher FW-FA inside the left superior longitudinal fasciculus (Figure 2). On the other hand, SMC and CI displayed higher FW index than HC, while CI showed higher FW index than SMC (Figure 3). Significant voxel-based correlations with nISD were found mainly in the anterior/posterior limb of internal capsule. PSMD analysis in the whole brain showed significant differences across groups.Discussion
The FW correction algorithm for DTI is able to minimize the inaccuracies associated with partial volume effects, and we previously showed it can improve the accuracy of DTI-related metrics in aging populations15,16. On the other hand, PSMD is a robust marker for cerebral small vessel disease based on DTI and has been used previously to study cognitive functions17.Here, both FW-DTI (for multi-shell DTI) and PSMD analysis showed microstructural WM differences across HC, SMC, and CI cohorts. Large differences across groups were found primarily in the corpus callosum; additionally, both methods were able to distinguish CI from SMC. Moreover, FW-DTI found significant voxel-based correlations with the nISD score.
Conclusions
In conclusion, the use of multi-shell FW-DTI and PSMD may provide novel insight into sub-voxel neurodegenerative processes and might warrant further exploration in more SMC and CI patients.Acknowledgements
Arizona Alzheimer’s consortium and Barrow neurological foundationReferences
1. Geda, Y. E. Mild cognitive impairment in older adults. Curr Psychiatry Rep 14, 320–7 (2012).
2. Petersen, R. C. et al. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol 56, 303–8 (1999).
3. Petersen, R. C. et al. Current concepts in mild cognitive impairment. Arch Neurol 58, 1985–92 (2001).
4. Balash, Y. et al. Subjective memory complaints in elders: depression, anxiety, or cognitive decline? Acta Neurol Scand 127, 344–50 (2013).
5. Jessen, F. et al. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimers Dement 10, 844–52 (2014).
6. Power, M. C. et al. Association of white matter microstructural integrity with cognition and dementia. Neurobiol Aging 83, 63–72 (2019).
7. https://dipy.org/documentation/1.0.0./examples_built/reconst_fwdti.
8. Baykara, E. et al. A Novel Imaging Marker for Small Vessel Disease Based on Skeletonization of White Matter Tracts and Diffusion Histograms. Ann Neurol 80, 581–92 (2016).
9. Schaefer, S. Y., Dibble, L. E. & Duff, K. Efficacy and Feasibility of Functional Upper Extremity Task-Specific Training for Older Adults With and Without Cognitive Impairment. Neurorehabil Neural Repair 29, 636–44 (2015).
10. Tournier, J. D. et al. MRtrix3: A fast, flexible and open software framework for medical image processing and visualisation. NeuroImage vol. 202 Preprint at https://doi.org/10.1016/j.neuroimage.2019.116137 (2019).
11. Jenkinson, M., Beckmann, C. F., Behrens, T. E., Woolrich, M. W. & Smith, S. M. FSL 1. Neuroimage 62, (2012).
12. http://stnava.github.io/ANTs/.
13. Garyfallidis, E. et al. Dipy, a library for the analysis of diffusion MRI data. Front Neuroinform 8, 8 (2014).
14. https://afni.nimh.nih.gov.
15. Bergamino, M., Walsh, R. R. & Stokes, A. M. Free-water diffusion tensor imaging improves the accuracy and sensitivity of white matter analysis in Alzheimer’s disease. Sci Rep 11, (2021).
16. Bergamino, M. et al. Sex Differences in Alzheimer’s Disease Revealed by Free-Water Diffusion Tensor Imaging and Voxel-Based Morphometry. Journal of Alzheimer’s Disease 85, (2021).
17. Deary, I. J. et al. Brain Peak Width of Skeletonized Mean Diffusivity (PSMD) and Cognitive Function in Later Life. Front Psychiatry 10, 524 (2019).
Figures