5267
Optimized B0 and B1+ insensitive RF pulses for non-selective T2 and diffusion preparation1Department of Bioengineering, Stanford University, Stanford, CA, United States, 2Department of Radiology, Stanford University, Stanford, CA, United States, 3Department of Electrical Engineering, Stanford University, Stanford, CA, United States
Synopsis
Keywords: New Signal Preparation Schemes, Data Acquisition
The performance of conventional T2 or diffusion preparation using linear phase pulses or adiabatic pulses is limited by the B1+ and B0 field inhomogeneities, particularly relevant in body imaging. In this work, we proposed a numerical optimization strategy to design RF pulse pairs for non-selective T2 or diffusion preparation that perform robustly over a wide range of B1+ and B0 variations. Phantom and in vivo experiments compared the performance of preparation using the optimized pulses to that of linear-phase and adiabatic pulses.Introduction
Conventional T2 or diffusion contrast preparation uses single- or twice-refocused schemes with linear-phase pulses1,2. Their performance is limited by the nonuniform RF transmit field (B1+) which commonly presents in body imaging applications such as liver and breast MRI. Though the problem could be mitigated by adiabatic pulses, adiabatic excitation pulses have low RF bandwidth and are therefore sensitive to B0 inhomogeneity3. In this work, we propose a strategy to design RF pulse pairs for non-selective T2 or diffusion preparation with robustness over a wide range of B1+ and B0 inhomogeneity. Pulses were designed using numerical optimization with a fixed duration, and target range of B0 and B1+ variations4. The prepared magnetization satisfies non-linear phase CPMG condition by using an “identical-phase” pulse pair5,6. With the “identical-phase” pulses, the 90° and 180° phases allowing more flexibility in performance, and the phase can be corrected by the tip-up -90° pulse. Phantom and in vivo experiments compared the performance of the optimized pulses to linear-phase and adiabatic excitation/refocusing schemes.Methods
The single- and twice-refocused preparations sequences and the optimization pipelines used to obtain the pulse pair are shown in Figure 1. Since the single- and twice-refocused prepared signal have distinct behaviors due to multiple magnetization pathways and the CPMG condition, we adopted different design strategies.For twice-refocused preparation, repeating a 90°-180° pulse pair cancels the phase contribution. Therefore the 90° and 180° pulses can be optimized independently. However, to achieve a spin echo, care needs to be taken to determine the isocenters of the non-symmetric optimized pulses and apply the correct timing. To find the isocenter, we simulated the preparation by applying different time offsets to the excitation pulse and chose the timing that most closely matched the desired profile.
For single-refocused preparation, we used an identical-phase pulse pair5. The identical-phase pulse pair requires that the magnetization after excitation should be along the rotation axis of refocusing pulse. With this condition met, the phase after the 90° pulse is unaffected by the 180° pulse so that the -90° can correct it reversely as a tip-up. We optimized the refocusing pulse first and used the resultant transverse phase as the desired excitation phase profile to achieve the “identical-phase” condition.
All pulse optimizations were initialized with a BIR-4 pulse with the same flip angle3. Duration of pulse=4.8ms, time step=48us, B1+=0.6:0.1:1.2. Desired excitation bandwidth is 1000Hz for 180° pulse, and is 800Hz for 90° pulses. B1max=0.2G. Minimization problems shown in Figure 1 were solved in MATLAB with the non-linear constrained minimization algorithm.
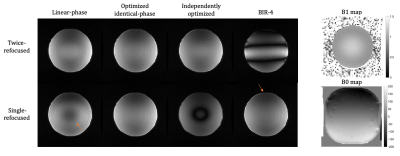
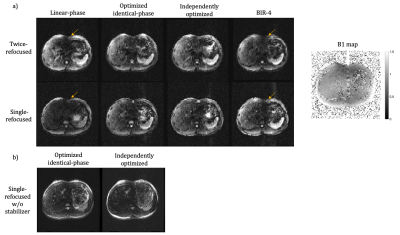
Phantom and in vivo experiments were performed at 3T on a GE Healthcare (Milwaukee, WI) Signa Premier to validate the preparation performance of the optimized pulses and compared with linear-phase pulses, and BIR-4 pulses with the same flip angles and durations. Excitation time was shifted by 2.3 ms in the twice-refocused preparation. All scans used a 3D FLASH readout. For the phantom experiment, a uniform agar ball was scanned using the same T2 preparation above. FOV=25x25cm, TEprep=60ms, TR=2000ms, ETL=64. The shim was perturbed to impart ±200Hz across the FOV. For the in vivo experiment, an axial abdomen scan with T2 preparation was performed on a healthy volunteer following IRB approval and informed consent. FOV=40x40cm, slice thickness=5mm, matrix=128x128x32, ETL=128, TR=2000 ms, TEprep=60ms, breath-hold time per scan=36seconds. Stabilizers for phase-insensitive diffusion preparation was on2.
Results
The optimized pulses and the simulated frequency profiles are shown in Figure 2. The frequency profile at the end of preparation using optimized pulses pairs compared with linear-phase and BIR-4 pulses are presented in Figure 3.Comparisons in phantom and axial abdomen with T2 preparation using optimized pulses, BIR-4 and linear-phase pulses are shown in Figure 4 and 5.
Discussion
In this work, a strategy of designing B0 and B1+ insensitive RF pulses pairs for T2 or diffusion preparation is proposed. An identical-phase pulse pair was designed for single-refocused preparation so that after the 90°-180° pulse pair, the magnetization is aligned with the resultant phase of the -90° pulse to maximize the tip-up signal. When designing the desired frequency profile for 90° pulses, phases for all B1+ values need to be simulated separately and integrate into the desired profile accordingly. Though the identical phase pulse pair was designed for single-refocused preparations, it can also be easily used for twice-refocused preparations, with a result that is less sensitive to phase errors.Though experiments were performed using T2 preparation, the proposed method can be extended to diffusion preparation. In this case, twice-refocused preparation will have a longer TE, but will help correct for gradient eddy currents. Another trade-off between single- and double-refocused preparations is the specific absorption rates (SAR). Single-refocused tends to have a lower SAR. The proposed method could achieve a uniform, high-bandwidth tip-up signal in both schemes.
SAR of the optimized pulses is comparable to BIR-4 with the same B1max. In ultra-high field systems (e.g., 7T), SAR penalties could be incorporated into the cost function to find the balance between B0/B1+ insensitivity and SAR.
Conclusion
A strategy of designing B0 and B1+ insensitive RF pulse pairs for non-selective T2 or diffusion preparation is proposed and demonstrated to achieve much better robustness to B0 and B1+ variations.Acknowledgements
NIH R01-EB009055References
1. Gibbons EK, Vasanawala SS, Pauly JM, Kerr AB. Body diffusion‐weighted imaging using magnetization prepared single-shot fast spin echo and extended parallel imaging signal averaging. Magnetic Resonance in Medicine 2017; 79:3032-44. doi:10.1002/mrm.26971.
2. Zhang Q, Coolen BF, Versluis MJ, Strijkers GJ, Nederveen AJ. Diffusion-prepared stimulated-echo turbo spin echo (DPsti-TSE): An eddy current-insensitive sequence for three-dimensional high-resolution and undistorted diffusion-weighted imaging. NMR in Biomedicine 2017;30. doi:10.1002/nbm.3719.
3. Staewen RS, Johnson AJ, Ross BD, Parrish T, Merkle H, Garwood M. 3-D flash imaging using a single surface coil and a new adiabatic pulse, bir-4. Investigative Radiology 1990; 25:559–567. doi: 10.1097/00004424-199005000-00015.
4. Moore J, Jankiewicz M, Zeng H, Anderson AW, Gore JC. Composite RF pulses for B1+ insensitive volume excitation at 7 tesla. Journal of Magnetic Resonance 2010; 205:50-62. doi: 10.1016/j.jmr.2010.04.002.
5. Lee PK, Yoon D, Sandberg JK, Vasanawala SS, Hargreaves BA. Volumetric and multispectral D near metallic implants using a non-linear phase carr-purcell-meiboom-gill diffusion preparation. Magnetic Resonance in Medicine 2022; 87:2650–2666. doi: 10.1002/mrm.29153.
6. Pauly JM, Wong EC. Non- linear phase RF pulses for reduced dynamic range in 3D rare imaging. In: Proceedings of the 9th Annual Meeting of ISMRM Glasgow, Scotland, UK; 2001. p. 688.
Figures
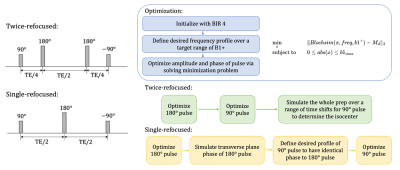
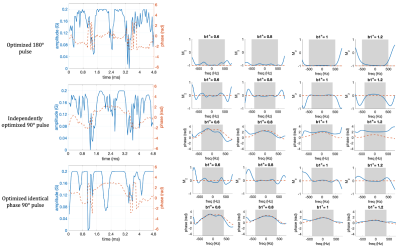
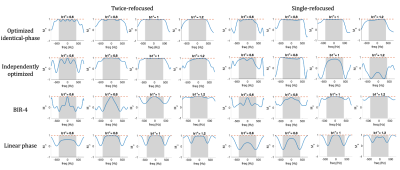
Figure 2. Optimized pulses, and the simulated Mz and phase profiles of each pulse. Shaded area represents the desired RF bandwidth (1000Hz for 180° pulse and 800Hz for 90° pulses). Dashed orange line is the desired Mz profile (-1 for 180° pulse and 0 for 90° pulse) and transverse plane phase of the optimized 180 pulse. The 180° pulse is designed first. The identical-phase 90° pulse shows a good consistency to the transverse plane phase of 180° compared to the independently designed one.