5266
Influence of Gradient Polarity in Magnetic Resonance Spectroscopy at 3T using HERMES
Antonia Susnjar1, Antonia Susnjar2, Gianna Nossa3, and Ulrike Dydak3,4
1Biomedical Engineering, Purdue University, West Lafayette, IN, United States, 2Purdue University, West Lafayette, IN, United States, 3School of Health Sciences, Purdue University, West Lafayette, IN, United States, 4Department of Radiology and Imaging Sciences, Indiana School of Medicine, Indianapolis, IN, United States
1Biomedical Engineering, Purdue University, West Lafayette, IN, United States, 2Purdue University, West Lafayette, IN, United States, 3School of Health Sciences, Purdue University, West Lafayette, IN, United States, 4Department of Radiology and Imaging Sciences, Indiana School of Medicine, Indianapolis, IN, United States
Synopsis
Keywords: Data Acquisition, Data Acquisition
To overcome acquisition downfalls of HERMES edited magnetic resonance spectroscopy at 3T, we have conducted critical optimization for four clinically relevant brain regions in neurological disorders by finding the optimal voxel rotation that affects gradient order to enhance data quality and reproducibility.Introduction
Have you ever had to discard data in your study where participants’ recruitment is limited? In our large post-traumatic stress disorder study, we had to discard over fifty percent of edited MRS data due to the poor SNR (<3), spurious echos, and dephasing of unwanted coherences. These artifacts could arise from insufficient, anisotropic B0 field distribution, suboptimal slice selection localization, and of course water and air in sinuses and mouth. To reduce unwanted artifacts, robust volume localization is necessary for both single voxel edited and unedited MRS 3. Here, we are focusing on data acquisition guidelines for edited MRS, specifically HERMES at Siemens Prisma 3T in hippocampus, insula, anterior cingulate cortex, dorsolateral prefrontal cortex, and insula. Identifying optimal voxel position and angulation that affects gradient order is crucial for acquiring high quality data and minimizing out of volume spurious signals as seen in Figure 1.Methods
The study was approved by the Institutional Review Boards (IRBs) of Purdue University and informed consent was obtained. Twenty participants (16 males, 4 females, age: 33.7±8.3) underwent an hour-long brain scan with a whole-body 3T Siemens PRISMA MRI system (Siemens Healthineers, Erlangen, Germany). A vendor-supplied 64-channel receiver head coil was used across all participants. Four brain regions: anterior cingulate cortex (ACC), dorsolateral prefrontal cortex (DLPFC), insula, and hippocampus (hippo) have been investigated as a part of the larger study assessing alterations in neurometabolic profile of post-traumatic stress disorder. 3D T1-weighted images (MPRAGE, TR/TE = 2300/2.98 ms, slice thickness = 1.2 mm, field-of-view = 256 × 256 mm2) were acquired to select a volume-of-interest (VOI). Edited MRS was acquired using Hadamard Encoding and Reconstruction of MEGA-Edited Spectroscopy (HERMES)2 with TE/TR:80/2000ms, 256 avgs, and VOI:40x25x27mm3. Data was analyzed and visualized for spectral evaluation in Gannet. Systematic variations in VOI rotation that affect gradient order for localization were evaluated for optimal acquisition. Data was discarded if it did not meet the standards of consensus papers as well as visual inspection where frequency drift, spurious echos, lipid contamination, and motion were observed in fitted spectra.Results
Optimal slice position, voxel rotation that resulted in different slice selection gradient order was determined for four brain regions of interest. Figure 3 illustrates where parameters can be found on Siemens Prisma 3T scanner, while Figure 4 illustrates our recommended findings with included image reference for all four brain regions.Slice selection order could be determined from combination of Sagittal (S), Coronal (C), and Transverse (T) order.
For ACC we found the most consistent results in S-T-C order and a VOI rotation >50º.
In DLPFC the T-C-S order or S-T-C order are recommended.
In hippocampus S-T-C order with VOI rotation >60º or T-S-C is optimal.
Lastly, in insula S-T-C or S-C-T order at positive angles is recommended.
Discussion and Conclusion
Optimal voxel rotation and slice selection order allowed us to significantly decrease the amount of data we discarded due to spurious echoes or artifacts as shown in Figure 2. Our future studies will include simulating gradients and cross-checking our findings with other edited sequences across GE and Siemens scanners.Acknowledgements
We would like to thank Purdue University Northwest, Weldon School of Biomedical Engineering, Leslie A. Geddes Graduate Fellowship, and Bottorff Fellowship for their funding and support.References
1. Ernst, T, and L Chang. “Elimination of artifacts in short echo time H MR spectroscopy of the frontal lobe.” Magnetic resonance in medicine vol. 36,3 (1996): 462-8.
2. Chan, K. L., Oeltzschner, G., Saleh, M. G., Edden, R., & Barker, P. B. (2019). Simultaneous editing of GABA and GSH with Hadamard-encoded MR spectroscopic imaging. Magnetic resonance in medicine, 82(1), 21–32. https://doi.org/10.1002/mrm.27702
3. Shams, Z, Klomp, DWJ, Boer, VO, Wijnen, JP, Wiegers, EC. Identifying the source of spurious signals caused by B0 inhomogeneities in single-voxel 1H MRS. Magn Reson Med. 2022; 88: 71- 82. doi:10.1002/mrm.29222
Figures
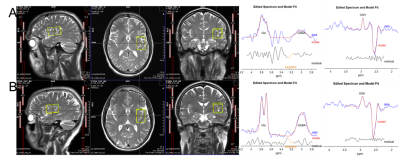
Figure 1. Optimization of data acquisition such as finding optimal voxel rotation, and slice excitation order is crucial for acquiring reproducible data.
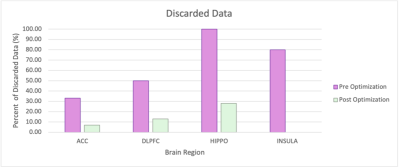
Figure 2. Plot of discarded data pre and post optimization.
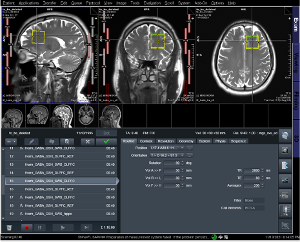
Figure 3. Voxel rotation and slice order information on Siemens Prisma scanner.
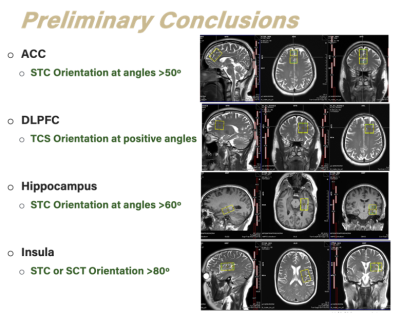
Figure 4. Preliminary conclusion and voxel position, rotation, and slice order for four clinically relevant brain regions.
DOI: https://doi.org/10.58530/2023/5266