5256
Multicontrast ZTE Neuroimaging with T1-Shuffling
Tobias C Wood1 and Emil Ljungberg1,2
1King's College London, London, United Kingdom, 2Lund University, Lund, Sweden
1King's College London, London, United Kingdom, 2Lund University, Lund, Sweden
Synopsis
Keywords: Image Reconstruction, Image Reconstruction
We show that multiple clinical contrasts can be obtained from silent Zero Echo Time scans by using preparation pulses and a physics-informed subspace "T1-shuffling" reconstruction approach. This models and removes the effect of T1-recovery during the readout segment, which would otherwise wash out the desired contrast.Introduction
Zero Echo-Time (ZTE) is a unique form of MRI with advantages including near-silent acoustics, rapid acquisition, and sensitivity to short T2 materials such as bone1. A naïve ZTE sequence can only acquire Proton Density or T1-weighted images due to its gradient-echo nature. Other contrasts including T2-weighting, diffusion and Magnetization Transfer have been demonstrated through the use of preparation modules, however the repeated sampling of the center of k-space in every TR washes out the desired contrast due to T1 recovery during a segment2,3,4. Clinical contrasts such as T2-FLAIR and Double Inversion Recovery (DIR), which require capturing magnetization in a very particular state, have hence not yet been demonstrated with a ZTE acquisition.In order to address this we utilised a sub-space reconstruction approach. Discovered independently in the context of T2-shuffling and MR fingerprinting5,6, the sub-space approach projects acquired k-space data onto a number of basis vectors that describe the evolution of the magnetization during the sequence7. The reconstructed basis images can then be linearly combined using the basis vectors to produce an image containing the contrast at one particular timepoint of the magnetization evolution. We exploited this to produce MP-RAGE, DIR, T2-weighted, and T2-FLAIR type contrasts with a ZTE readout at clinically useful resolutions and scan-times.
Methods
All images were acquired on a 3T scanner equipped with a 48-channel head coil (GE Healthcare) and reconstructions utilized the RIESLING toolbox8. Obtaining high-quality images required a circuitous and torturous optimization process due to the interlinked nature of the acquisition and reconstruction.We first fixed several parameters to clinically useful values - 1mm isotropic resolution, 220 isotropic matrix size, and readout bandiwdth ±31.25kHz, resulting in a TR of 2ms. We then noted that DIR, T2w, and T2-FLAIR are typically acquired with Fast Spin Echo sequences, which have long periods of dead-time to allow for T1-recovery but a short, high SNR readout train where the magnetization is in the transverse plane. In contrast, ZTE readout segments are usually much longer (512 spokes per segment is typical) and the magnetization is mostly longitudinal (flip-angle < 5°). We hence placed read-out segments where there would normally be dead-time, but with a low 2° flip-angle to not disturb T1-recovery.
We next observed that between the first and second inversion pulses in the DIR sequence the magnetization evolution resembles that during the MP-RAGE sequence. We hence could extract the MP-RAGE contrast from the DIR scan "for free", allowing more time during the protocol to be spent on the DIR sequence to improve SNR. To enable the same trick with the T2-FLAIR and T2w images we replaced the inversion pulse in the FLAIR sequence with a combined T2-prep and inversion pulse, and inserted additional T2-prep pulses between each readout segment to increase the overall amount of T2-weighting in the sequence.
Subspace methods, and compressed-sensing techniques in general, require incoherence in the data to prevent artefacts. A truly incoherent acquisition such as Poisson-Disk sampling is not possible with ZTE due to the need to minimise gradient steps between spokes. Instead, we used a hybrid approach with a phyllotaxis interleaf for the trajectory within a segment9, but distributed the starting point of the interleaves using 2D golden means10, to at least introduce incoherency between segments.
The final T1-weighted scan used 480 spokes-per-segment, with 2 segments after the first inversion pulse and 4 after the second inversion pulse, with a scan-time of 10 minutes. The MP-RAGE and DIR contrasts were formed from the 600th and 1100th TR respectively. The T2-weighted scan used 600 spokes-per-segment, with 1 segment after the combined T2 & inversion pulse and then 4 further segments with T2 pulses before each one. The TE of the preparation pulses was 100 ms. The T2-weighted and FLAIR contrasts were calculated directly after the first and second prep pulses. Figure 1 shows simulations of the sequences.
Both scans were compressed to 12 channels11, sensitivity maps extracted from low-resolution calibration scans, and then reconstructed using 8 basis images and 10 iterations of the preconditioned Primal-Dual Hybrid-Gradient optimizer12 with a Locally-Low-Rank regularizer5. Reconstructions utilised approximately 256 gigabytes of memory and took around 1 hour using 16 cores.
Results
Figures 2 and 3 show the basis vectors and reconstructed basis images for both scans. In both cases, there are three basis images that contain the majority of the signal. Figure 4 shows slices through the final MP-RAGE, DIR, T2-weighted and T2-FLAIR contrasts. The MP-RAGE and DIR images show excellent resolution and high SNR. The T2-weighted and T2-FLAIR show the overall correct contrast although less White Matter/Grey Matter contrast than is typical with FSE acquisitions.Conclusion
We have shown that it is possible to generate typical clinical contrasts from a silent ZTE scan in a feasible scan time using a subspace or "T1-shuffling" approach.Acknowledgements
This work was funded in part by the CHDI foundation.References
- Ljungberg, Emil, Nikou Damestani, Tobias C Wood, David J Lythgoe, Fernando Zelaya, Steven C R Williams, Ana Beatriz Solana, Gareth J Barker, and Florian Wiesinger. ‘Silent Zero TE MR Neuroimaging: Current State-of-the-Art and Future Directions’. Progress in Nuclear Magnetic Resonance Spectroscopy, 2021, 21. https://doi.org/10.1016/j.pnmrs.2021.03.002.
- Solana, Ana Beatriz, Anne Menini, Laura I. Sacolick, Nicolas Hehn, and Florian Wiesinger. ‘Quiet and Distortion-Free, Whole Brain BOLD FMRI Using T2-Prepared RUFIS’. Magnetic Resonance in Medicine 75, no. 4 (April 2016): 1402–12. https://doi.org/10.1002/mrm.25658.
- Yuan, Jianmin, Yuxin Hu, Anne Menini, Christopher M. Sandino, Jesse Sandberg, Vipul Sheth, Catherine J. Moran, et al. ‘Near‐silent Distortionless DWI Using Magnetization‐prepared RUFIS’. Magnetic Resonance in Medicine, 29 November 2019. https://doi.org/10.1002/mrm.28106.
- Wood, Tobias C., Nikou L. Damestani, Andrew J. Lawrence, Emil Ljungberg, Gareth J. Barker, Ana Beatriz Solana, Florian Wiesinger, and Steven C.R. Williams. ‘Silent Myelin-Weighted Magnetic Resonance Imaging’. Wellcome Open Research 5 (13 August 2020): 74. https://doi.org/10.12688/wellcomeopenres.15845.2.
- Tamir, Jonathan I, Martin Uecker, Weitian Chen, Peng Lai, Marcus T Alley, Shreyas S Vasanawala, and Michael Lustig. ‘T2 shuffling: Sharp, multicontrast, volumetric fast spin‐echo imaging’ 77 (2017): 180–95.
- Assländer, Jakob, Martijn A. Cloos, Florian Knoll, Daniel K. Sodickson, Jürgen Hennig, and Riccardo Lattanzi. ‘Low Rank Alternating Direction Method of Multipliers Reconstruction for MR Fingerprinting: Low Rank ADMM Reconstruction’. Magnetic Resonance in Medicine 79, no. 1 (January 2018): 83–96. https://doi.org/10.1002/mrm.26639.
- Wang, Xiaoqing, Zhengguo Tan, Nick Scholand, Volkert Roeloffs, and Martin Uecker. ‘Physics-Based Reconstruction Methods for Magnetic Resonance Imaging’. Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences 379, no. 2200 (28 June 2021): 20200196. https://doi.org/10.1098/rsta.2020.0196.
- Wood, Tobias, Emil Ljungberg, and Florian Wiesinger. ‘Radial Interstices Enable Speedy Low-Volume Imaging’. Journal of Open Source Software 6, no. 66 (7 October 2021): 3500. https://doi.org/10.21105/joss.03500.
- Ljungberg, Emil, Tobias C. Wood, Ana Beatriz Solana, Steven C. R. Williams, Gareth J. Barker, and Florian Wiesinger. ‘Motion Corrected Silent ZTE Neuroimaging’. Magnetic Resonance in Medicine, 5 April 2022, mrm.29201. https://doi.org/10.1002/mrm.29201.
- Chan, Rachel W., Elizabeth A. Ramsay, Charles H. Cunningham, and Donald B. Plewes. ‘Temporal Stability of Adaptive 3D Radial MRI Using Multidimensional Golden Means’. Magnetic Resonance in Medicine 61, no. 2 (February 2009): 354–63. https://doi.org/10.1002/mrm.21837.
- Huang, Feng, Sathya Vijayakumar, Yu Li, Sarah Hertel, and George R. Duensing. ‘A Software Channel Compression Technique for Faster Reconstruction with Many Channels’. Magnetic Resonance Imaging 26, no. 1 (January 2008): 133–41. https://doi.org/10.1016/j.mri.2007.04.010.
- Ong, Frank, Martin Uecker, and Michael Lustig. ‘Accelerating Non-Cartesian MRI Reconstruction Convergence Using k-Space Preconditioning’. IEEE Transactions on Medical Imaging 39, no. 5 (May 2020): 1646–54. https://doi.org/10.1109/TMI.2019.2954121.
Figures
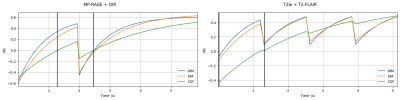
Figure 1 Simulations of the longitudinal magnetization evolution during the combined MP-RAGE & DIR sequence (left) and the combined T2w & T2-FLAIR sequence (right), for Grey Matter, White Matter and CSF. Black vertical lines mark the timepoints where the images were reconstructed to yield the correct contrasts.

Figure 2 Basis vectors (left) and reconstructed basis images (right) for the combined MP-RAGE & DIR sequence. The images are presented on a log-magnitude scale due to differing intensity scales.

Figure 3 Basis vectors (left) and reconstructed basis images (right) for the combined T2w & T2-FLAIR sequence. The images are presented on a log-magnitude scale due to differing intensity scales.

Figure 4 Final reconstructed images for the different contrasts from the two sequences.
DOI: https://doi.org/10.58530/2023/5256