5238
Colloidal Cell Mimics as Reference System for Diffusion MRI Experiments
Henrik zu Jeddeloh1,2, Dominik Ludwig1, Julian Rauch1,2, Frederik Laun3, Mark Ladd1,2,4, Karel Klika5, and Tristan Anselm Kuder1,2
1Division of Medical Physics in Radiology, German Cancer Research Center (DKFZ), Heidelberg, Germany, 2Faculty of Physics and Astronomy, Heidelberg University, Heidelberg, Germany, 3Institute of Radiology, University Hospital Erlangen, Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU), Erlangen, Germany, 4Faculty of Medicine, Heidelberg University, Heidelberg, Germany, 5Molecular Structure Analysis, German Cancer Research Center (DKFZ), Heidelberg, Germany
1Division of Medical Physics in Radiology, German Cancer Research Center (DKFZ), Heidelberg, Germany, 2Faculty of Physics and Astronomy, Heidelberg University, Heidelberg, Germany, 3Institute of Radiology, University Hospital Erlangen, Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU), Erlangen, Germany, 4Faculty of Medicine, Heidelberg University, Heidelberg, Germany, 5Molecular Structure Analysis, German Cancer Research Center (DKFZ), Heidelberg, Germany
Synopsis
Keywords: Phantoms, Diffusion/other diffusion imaging techniques, Cell Mimics, Polymerized Spheres
In order to verify modern diffusion MRI sequences, a well-defined reference system consisting of polymerized, hollow microspheres with a hole in the surface is synthesized. This reference system can model the diffusion properties of cells with varying size1,3 and membrane permeability2. To produce these cell mimics3, tripropyleneglycol-monomethylether monomer was mixed with an ammonia solution with polystyrene particles to initiate a nucleation process. The droplets were inflated in an NaOH solution and then hardened under UV exposure. Lastly, the polystyrene particles were dissolved. Scanning Electron Microscope images and DWI measurements were acquired to verify the quality of the cell mimics.Introduction
Specialized diffusion MRI sequences yield insight into the microscopic structure of cells, for example regarding cell size and membrane permeability1,2,3. It is, however, difficult to verify the effectiveness of these measurements, which necessitates the generation of a well-defined reference system for verification experiments. There are different approaches in literature such as the use of polyamide fibers, glass capillaries or nanolithography. Most of these techniques can only model either the intra- or the extracellular compartment. A recent approach uses photo-polymerization of a monomer to create hollow microspheres filled with an aqueous solution4,5. The permeability of a cell’s membrane can be emulated through a hole in the the cell mimic. The aim of this work was the optimization of this polymerization approach as a model system for advanced DWI sequences such as approaches for measuring cell size and shape distributions1 or membrane permeability. While cell mimics with a diameter below 5 µm can be easily synthesized, larger diameters are more difficult to achieve. Therefore, an optimization was performed to generate a larger fraction of diameters > 5 µm resembling typical cell sizes. Scanning electron microscopy (SEM) and diffusion MR measurements were then used to verify the successful production of the cell mimics.Materials & Methods
The synthesis of the cell mimics4,5 was started by adding tripropyleneglycol-monomethylether (TPM) to an ammonia solution (pH ≈ 10), where the TPM monomers created a cross-linking network and form droplets with a diameter between 2 and 4 µm. The inflation of the TPM droplets was initiated through the introduction of a strong NaOH solution (between 150 and 200 mmol), which resulted in the hydrolysis of the internal structure of the TPM droplets. The resulting osmotic pressure led to an inflation of the droplets to a diameter of 5 to 8 µm. With the addition of a photo initiator, the UV polymerization can follow, resulting in a hard outer shell of the droplet. The resulting cell mimics are hollow spheres with their internal volume filled with an aqueous solution.For cell mimics with a hole, negatively charged polystyrene (PS) particles were utilized as a nucleation site for the TPM droplets4,5. After the UV polymerization, the PS particles need to be dissolved with toluene such that a hole remains. To verify the cell mimics with hole, SEM images were obtained. To optimize the cell mimic size, various TPM monomer injection volumes as well as nucleation times were tested. To maximize the degree of inflation, and therefore diameter increase, an increased concentration of NaOH was chosen.
Diffusion-weighted measurements (DWI) were acquired using a 9.4T Bruker scanner with $$$G_{max}$$$ = 660 mT/m, $$$\delta$$$ = 6 ms, $$$\Delta$$$ = 11.84 ms and b-values from 0 to 15396 s/mm2.
Results
Using the optimized synthesis process, cell mimic diameters of up to 9 µm could be produced. The size distribution of the spheres is rather inhomogeneous, with some cell mimics measuring as small as 3 µm in diameter (Fig. 1). A detailed SEM image of a single cell mimic with hole is shown in Fig. 2. DWI measurements to verify the synthesis using a batch of microspheres without hole (approx. diameter: 3 µm) and with hole (approx. diameter 6 µm) show higher signals with increasing b-values as compared to pure water samples (Fig. 3a). A biexponential decay of the signal is present, finally transitioning to a signal plateau at very high b-values due to motional narrowing for the cell mimic samples in contrast to pure water (Fig. 3b). While the cell mimics without hole are smaller compared to the ones with, the diffusion process inside the pores approaches the long-time limit for both cases. The faster signal decrease in Fig. 3a for the case with holes can thus be attributed to the exchange between the intra- and extraporal compartment.Discussion
The SEM images demonstrate the successful inclusion of the hole in the cell mimic. The presence of intra- and extraporal compartments as well as the effect of the introduction of holes could be verified using DWI measurements. Using the optimized synthesis parameters, larger cell mimics could be generated which are closer to typical cell sizes; these microspheres thus have the potential to be used for verification experiments considering the limited available gradient amplitude. However, the yield of the larger diameters is still limited.Besides further optimization, a filtering technique could be employed to retain only the spheres of larger diameter. This could also lead to a more homogeneous size distribution with an average diameter of approximately 8 µm; this is in the range of actual cell sizes and could thus lead to a more in vivo-like phantom.
Conclusion
In this work, the successful synthesis of cell mimics as a reference system for diffusion MR measurements could be demonstrated. Through the addition of a hole, various cell parameters can be modelled with this reference system, such as cell size, membrane permeability and packing density. The focus of this work was to generate larger cell mimics, which was successful to a degree. However, further work needs to be done in order to optimize the inclusion of the hole as well as achieve a more homogeneous size distribution of the cell mimics.Acknowledgements
No acknowledgement found.References
[1] Kuder et al., Diffusion Pore Imaging by Hyperpolarized Xenon-129 Nuclear Magnetic Resonance, Phys. Rev. Lett. 111(2):028101 (2013)
[2] Lasič et al., Apparent Exchange Rate Mapping with Diffusion MRI, Magn. Reson. Med 66(2):356-65 (2011)
[3] Jiang X et al., MR cell size imaging with temporal diffusion spectroscopy, Magn. Reson. Imaging 77:109-123 (2021)
[4] Xu et al., Transmembrane transport in inorganic colloidal cell-mimics, Nature 597:220–224 (2021)
[5] Silletta et al., Monitoring Molecular Transport across Colloidal Membranes. J Phys Chem B. 10;122(18):4931-4936 (2018)
Figures
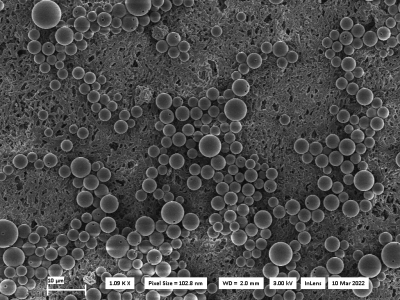
Figure 1: Scanning electron micrograph (SEM) image of multiple cell mimics with holes.

Figure 2: SEM image of a cell mimic with hole.
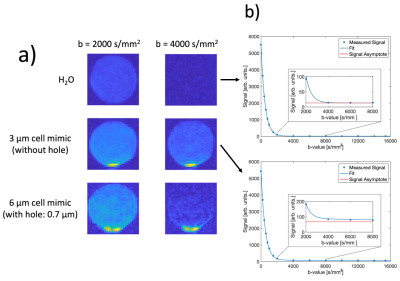
Figure 3: (a) Diffusion MRI for H2O and for a cell mimic solution for 2 b-values. The signal increase at the bottom of the tubes is due to sedimentation of the microspheres (b) DWI signal of H2O (top) and cell mimics (3 µm, without hole, bottom).
DOI: https://doi.org/10.58530/2023/5238