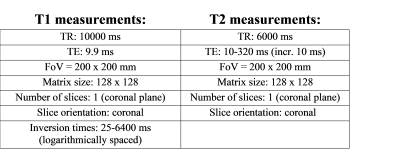5235
Relaxometry phantoms: Are there alternatives to paramagnetic additives?1Section on Experimental Radiology, Department of Diagnostic and Interventional Radiology, University of Tübingen, Tübingen, Germany
Synopsis
Keywords: Phantoms, Relaxometry
Phantoms used for relaxometry usually consist of a gelling agent doped with paramagnetic salt. However, since the use of paramagnetic additives can be disadvantageous for certain purposes, there is a great interest in finding other suitable substances. In this work, soy-lecithin-agar gels are presented and evaluated as an alternative phantom material for the construction of relaxometry phantoms with tissue-like relaxation times. They were found to work quite well. Soy-lecithin-agar gels are easy to prepare and allow independent adjustment of T1 and T2. Relaxation times of different tissues (muscle, liver, pancreas, kidney) could be successfully mimicked.Introduction
Phantoms that mimic the properties of biological tissue are indispensable tools for testing and optimizing quantitative MRI techniques.1 Their composition and production have become an important area of research. In particular, phantoms that mimic the relaxation times (T1, T2) of biological tissue are attracting considerable interest. Interestingly, there are very few approaches to the fabrication of phantoms with tissue-like relaxation times T1 and T2. Relaxometry phantoms proposed so far are typically two-component mixtures consisting of a gelling agent (e.g. agar, agarose) doped with a paramagnetic salt (e.g. MnCl2, GdCl3, NiCl2).2-8 Here, the paramagnetic salts generally serve as a T1-modifier and the gelling agent as a T2-modifier. Knowing the relaxivities (r1, r2) of each component, one can design a phantom material that has the desired relaxation times. The required concentrations of the respective substances can be calculated according to the following equations (1,2):5$$C_{a}= \frac{T2^{-1}-T2^{-1}_{w}-(\frac{r^{(b)}_{2}}{r^{(b)}_{1}})(T1^{-1}-T1^{-1}_{w})}{r^{(a)}_{2}-(\frac{r^{(b)}_{2}}{r^{(b)}_{1}})r^{(a)}_{1}}$$
$$C_{b}= \frac{T1^{-1}-T1^{-1}_{w}-(\frac{r^{(a)}_{1}}{r^{(a)}_{2}})(T2^{-1}-T2^{-1}_{w})}{r^{(b)}_{1}-(\frac{r^{(a)}_{1}}{r^{(a)}_{2}})r^{(b)}_{2}}$$
Ca,b: concentration of component a and b, respectively; r1(a,b), r2(a,b): relaxivities of component a and b, respectively; T1-1w, T2-1w: relaxation rates of pure water; T1-1,T2-2: relaxation rates to be achieved.
However, the presence of paramagnetic salts affects not only the relaxation times, but also the magnetic susceptibility of the phantom material.9,10 This could lead to disadvantageous effects especially using GRE sequences, since undesired magnetic field inhomogeneities occur dependent on the geometry and composition of the phantom. In addition, paramagnetic substances are toxic, which complicates the handling and production of these phantoms. Therefore, there is a certain interest in a phantom material that allows independent adjustment of relaxation times T1 and T2 without the use of paramagnetic salts. In this work, soy-lecithin-agar gels are presented and evaluated as an alternative material for the construction of phantoms with tissue-like relaxation times. A detailed study design is described in the next section.
Materials and Methods
Study designFirst, relaxivities (r1, r2) of soy-lecithin and agar at 3 Tesla were determined individually in aqueous soy-lecithin solutions and agar gels of different concentrations. Soy-lecithin and agar concentrations were varied between 0% and 6% by weight.
Second, it was investigated whether the relaxivities of soy-lecithin (and agar) remain stable or change when the two components are mixed in soy-lecithin-agar gels. For this, relaxivities of soy-lecithin were measured for a range of agar concentrations (0.5%, 1%, 1.5%, 2%). Similarly, relaxivities of agar were measured for each concentration of soy-lecithin (2%, 4%, 6%, 8%).
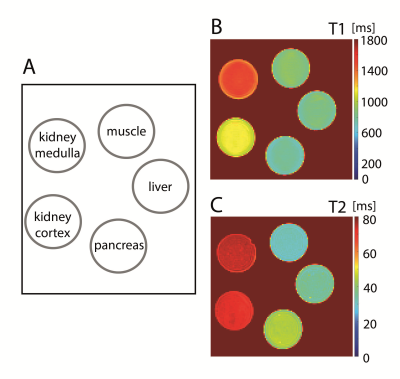
Finally, five test phantoms were prepared to mimic the relaxation times T1 and T2 of different tissues (muscle, liver, pancreas, kidney). Using the determined relaxivities and equation 1a,b, the appropriate concentrations of soy-lecithin and agar were calculated to achieve the desired T1 and T2 values.
Preparation of the samples
To prepare the soy-lecithin-agar gels, soy-lecithin and agar were first dissolved separately and then mixed. Soy-lecithin solutions were prepared by dissolving soy-lecithin (Carl Roth, Karlsruhe, Germany) in purified water under magnetic stirring at 650 rpm for 20 minutes. Agar gels were prepared by dissolving the appropriate amount of agar (Agar powdered, AppliChem Panreac, Darmstadt, Germany) in purified water using a microwave heater. The agar solution was then cooled and once the temperature of the solution dropped below 70°C, the soy-lecithin solution was added with gentle stirring to obtain a homogeneous mixture.
Data acquisition and analysis
All measurements were performed on a whole-body, 3.0T clinical MRI system (MAGNETOM Prismafit, Siemens Healthcare, Erlangen, Germany) using an 18-channel body array coil. The data were collected at room temperature of 21°C±1°C and processed offline with MATLAB (MathWorks, Natick, MA).
A TSE-based inversion-recovery pulse sequence was used for T1 measurements. Relaxation times T2 were assessed using a standard Carr-Purcell-Meiboom-Gill spin-echo pulse sequence (Acquisition parameters were included in Table 1). T1 and T2 values of each sample were determined from circular regions of interest in calculated T1 and T2 maps.
Results
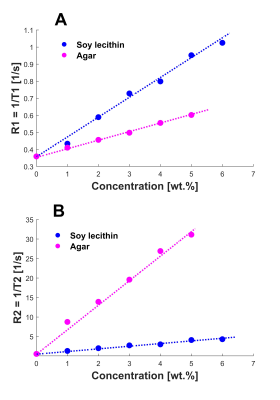
The relaxation rates for both the lecithin solutions and the agar gels showed a linear relationship with concentration (Figure 1). The relaxivities were determined to be r1,lecithin = 0.11 s-1·wt.%-1, r1,agar = 0.05 s-1·wt.%-1, r2,lecithin = 0.67 s-1·wt.%-1, and r2,agar = 5.73 s-1·wt.%-1.Measurements of the soy-lecithin-agar gels showed that relaxivities of soy-lecithin and agar remained almost stable and didn’t change significantly when the components were mixed. Only r1,agar increased with increasing soy-lecithin concentration (r1,agar = 0.05-0.09 s-1·wt.%-1), indicating that the effect of agar on T1 is enhanced in the presence of soy-lecithin. To account for this effect, the mean value r1,agar = 0.07 s-1·wt.%-1 was assumed for further analysis.
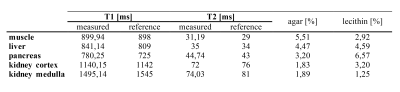
Figure 2 shows the T1 and T2 maps of the test phantoms mimicking relaxation times of different tissues. There was a very good agreement between measured and targeted relaxation times (Table 2).
Discussion and Conclusion
This work shows that soy-lecithin-agar gels represent an alternative phantom material for the preparation of relaxometry phantoms with tissue-like relaxation times. Soy-lecithin agar gels are easy to prepare and allow independent adjustment of T1 and T2 without the use of toxic substances. However, further work is needed to investigate reproducibility of the manufacturing process and the temporal stability of the gels.Acknowledgements
No acknowledgement found.References
1. Keenan KE, Ainslie M, Barker AJ, et al. Quantitative magnetic resonance imaging phantoms: A review and the need for a system phantom. Magn Reson Med. 2018;79:48-61.
2. Hattori K, Ikemoto Y, Takao W, et al. Development of MRI phantom equivalent to human tissues for 3.0-T MRI. Med Phys. 2013;40:032303.
3. Kato H, Kuroda M, Yoshimura K, et al. Composition of MRI phantom equivalent to human tissues. Med Phys. 2005;32:3199-3208.
4. Christoffersson JO, Olsson LE, Sjöberg S. Nickel-doped agarose gel phantoms in MR imaging. Acta Radiol. 1991;32:426-431.
5. Tofts PS, Shuter B, Pope JM. Ni-DTPA doped agarose gel--a phantom material for Gd-DTPA enhancement measurements. Magn Reson Imaging. 1993;11:125-133.
6. Mathur-De Vre R, Grimee R, Parmentier F, Binet J. The use of agar gel as a basic reference material for calibrating relaxation times and imaging parameters. Magn Reson Med. 1985;2:176-179.
7. Woletz M, Roat S, Hummer A, Tik M, Windischberger C. Technical Note: Human tissue-equivalent MRI phantom preparation for 3 and 7 Tesla. Med Phys. 2021;48:4387-4394.
8. Hellerbach A, Schuster V, Jansen A, Sommer J. MRI phantoms - are there alternatives to agar? PLoS One. 2013;8:e70343.
9. Hijnen NM, Elevelt A, Pikkemaat J, Bos C, Bartels LW, Grüll H. The magnetic susceptibility effect of gadolinium-based contrast agents on PRFS-based MR thermometry during thermal interventions. J Ther Ultrasound. 2013;1:8.
10. Hobson N, Polster SP, Cao Y, et al. Phantom validation of quantitative susceptibility and dynamic contrast-enhanced permeability MR sequences across instruments and sites. J Magn Reson Imaging. 2020;51:1192-1199.
11. Bojorquez JZ, Bricq S, Acquitter C, Brunotte F, Walker PM, Lalande A. What are normal relaxation times of tissues at 3 T? Magn Reson Imaging. 2017;35:69-80.
Figures