5233
Is the ASTM F2182 Gel Phantom Adequate for Determining Worst-Case RF-Induced Heating of Implanted Medical Devices?1MED Institute, West Lafayette, IN, United States
Synopsis
Keywords: Safety, Safety, Heating, RF-Induced Heating, MRI Safety Evaluations, Simulation
ASTM F2182 was developed to evaluate RF-induced heating near passive implanted medical devices by subjecting an implant within an average tissue-mimicking (i.e., electrical and thermal properties) phantom to controlled RF exposure. The purpose of this study was to determine if the ASTM F2182 phantom is adequate for determining worst-case RF-induced heating of implanted medical devices. Results from this study display that device temperature rises in the ASTM F2182 are significantly higher than in-vivo temperature rises, regardless of tissue-specific properties and local blood perfusion incorporation to the phantom, indicating that the ASTM gel phantom is adequate for RF-induced heating evaluations.Introduction
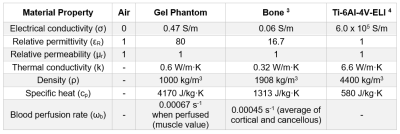
The body transmit radiofrequency (RF) coil of an MRI scanner creates a time-varying magnetic field (B1) that induces currents on conductive medical devices, which can result in heating of tissue near an implant. The extent of heating depends on a multitude of factors, such as the MRI system being used (1.5T/64 MHz or 3T/128 MHz), the type of transmit coil and scanning sequence (which impact RF energy deposition), location within the bore, and device characteristics (geometry, material, and positioning in the patient). Specifically, the device and the surrounding tissue electromagnetic (electrical conductivity, dielectric constant, and relative permeability) and thermal (specific heat capacity, thermal conductivity, and density) material properties and local blood perfusion impact RF-induced heating. A standard test method, ASTM F21821, was developed to evaluate RF-induced heating near passive implanted medical devices by subjecting an implant within an average tissue-mimicking (i.e., electrical and thermal properties) phantom to controlled RF exposure. The purpose of this study was to determine if the ASTM F2182 phantom is adequate for determining worst-case RF-induced heating of implanted medical devices.Methods
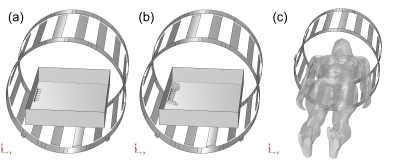
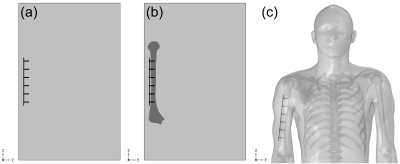
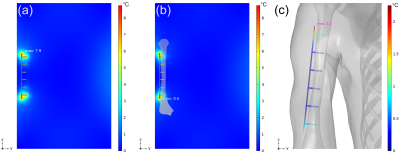
The ASTM F2182 test method was simulated in a 1.5T MRI system using MED Institute’s FDA qualified Medical Device Development Tool (MDDT)2 to determine RF-induced heating of two generic orthopedic implants, a hip stem and a bone plate with screws, in models with increasing anatomical fidelity. In order of increasing anatomical fidelity, the test method was simulated in an ASTM F2182 gel phantom (Figure 1a), an ASTM F2182 gel phantom with a bone inside of it (each with their respective material properties) and local blood perfusion (Figure 1b), and a virtual human anatomy (Duke) with tissue-specific material properties and local blood perfusion (Figure 1c). The material properties and blood perfusion rates3, 4 used in the simulations are shown in Figure 2. Positioning of each implant was the same for both phantom models and was clinically relevant in the virtual human anatomy (Figure 3). All aspects of the simulation (e.g., coil geometry, scanner frequency, driving voltage) other than those described above were maintained across all simulations. Maximum RF-induced temperature rise after 15 minutes of scanning at a whole phantom specific absorption rate (SAR) of 2 W/kg was determined for both implants in each of the varying anatomical fidelity models.Results
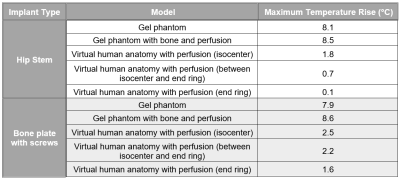
The maximum temperature rise in the tissue near the device for both implants in each model is shown in Figure 4 and temperature contour plots for the bone plate with screws are shown in Figure 5. For both implants, heating was slightly greater in the gel phantom with a bone and local blood perfusion than it was in the gel phantom alone (5% greater and 9% greater for the hip stem and the bone plate with screws, respectively). However, heating of the hip stem and of the bone plate with screws was significantly reduced in the virtual human anatomy when compared to the ASTM F2182 gel phantom (minimum of 78% and 68% reduction, respectively).Discussion
The ASTM F2182 test method was designed to evaluate worst-case RF-induced heating of passive implanted medical devices in an MR environment. By utilizing a phantom that is designed to lack convection and perfusion that help dissipate heat and to focus the electric field created by the MRI system, the test method represents extreme conditions and is considered to be conservative. The results of this study display that the use of a higher anatomical fidelity phantom, one that utilizes specific tissue properties and incorporates local blood perfusion, may in some cases result in slightly higher temperature rises than those in a gel phantom alone. However, the results also depict that the ASTM gel phantom with and without bone and perfusion are usually conservative compared to in-vivo heating and typically result in temperature rises that are similar to each other, but significantly greater than in-vivo. This indicates that the ASTM F2182 phantom is appropriate for determining a worst-case device/construct and the corresponding maximum temperature rise for RF-induced heating.Conclusion
The ASTM F2182 phantom does not replicate the tissue-specific electromagnetic and thermal properties of the tissue surrounding an implanted medical device and does not incorporate local blood perfusion. However, the results of this study indicate that a phantom with anatomical and physiological characteristics leads to a similar maximum temperature rise as the ASTM F2182 phantom and that the phantom is often highly conservative compared to in-vivo heating regardless of tissue-specific properties and local blood perfusion. Therefore, the ASTM F2182 phantom is adequate for determining worst-case RF-induced heating of implanted medical devices that can be used for MR Conditional labeling guidelines without incorporating device-specific surrounding tissues and blood perfusion in the phantom. However, virtual human anatomy modeling with perfusion may provide a more accurate assessment of the maximum RF-induced temperature rise of a medical device.Acknowledgements
No acknowledgement found.References
[1] ASTM F2182-19e2, Standard Test Method for Measurement of Radio Frequency Induced Heating On or Near Passive Implants During Magnetic Resonance Imaging, ASTM International, 2019.
[2] FDA, "MDDT Summary of Evidence and Basis of Qualification Decision for Virtual MRI Safety Evaluations of Medical Devices," 16 November 2021. [Online]. Available: https://www.fda.gov/media/154181/download. [Accessed 7 November 2022].
[3] P. Hasgall, F. Di Gennaro, C. Baumgartner, E. Neufeld, B. Lloyd, M. Gosselin, D. Payne, A. Klingenbock and N. Kuster, "IT'IS Database for thermal and electromagnetic parameters of biological tissues," Version 4.0, May 15, 2018, DOI: 10.13099/VIP21000-04-0. [Online]. Available: itis.swiss/database.
[4] "TIMET TIMETAL® 6-4 ELI Titanium Alloy (Ti-6Al-4V ELI; ASTM Grade 23)," MatWeb, [Online]. Available: www.matweb.com. [Accessed 16 April 2018].
Figures