5228
Development of an easy-manufacturable and cheap phantom for quality control of T1, T2 and fat fraction mapping.1Centre de Résonance Magnétiques des Systèmes biologiques UMR5536, CNRS/Université de Bordeaux, Bordeaux, France
Synopsis
Keywords: Phantoms, Quantitative Imaging, quality control
A quality control phantom was developed through a simple and easy protocol for T1, T2 and Fat fraction mapping. The phantom is constituted of 8 tubes containing Gd-DOTA or commercially available animal fat, each at various concentrations and stabilized in agarose gels. A 3D printed bubble trap was placed on top of the gels to limit the contrast agent dilution over time. The solutions are stable for at least 6 months and the manufacturing protocol is highly reproducible.INTRODUCTION
With the great interest in parametric mapping, quality controls of measurable quantities are now highly desirable. Several vendors sell phantoms containing T1 and/or T2 contrast agents in sealed containers; however they remain stable approximately 1 year. One last issue is the absence of commercial phantoms enabling to measure fat fraction. There is only one protocol that has been several times mentioned in literature, that employs peanut oil1. Unfortunately, vegan oil does not perfectly mimic human fat. Consequently, we developed a simple and easy to reproduce phantom for T1, T2 and Fat fraction (FF) measurements. The stability over time and reproducibility of the phantom was tested at 3T.MATERIALS and METHODS
Preparation of the phantom (Figure 1)For T1 and T2 analysis, 4ml of aqueous Gd-DOTA (Dotarem) were added to 4ml of 3% agarose gel solution to finally obtained 4 tubes at 0.2mM, 0.15mM, 0.05mM and 0.02mM Gd-DOTA in 1.5% of agar. Also, a last tube was filled with only 1.5% agar (0mM). The mixture was gently mixed to prevent the formation of bubbles before plunging the tubes into liquid nitrogen in order to rapidly freeze the solution. For FF analysis, a commercial porc fat (« Saindoux/lard ») volume of 2, and 4ml were added to 6 or 4ml agarose gel at 2-3% to finally obtained tubes with a volume FF of 25% and 50%, respectively. Also, a last tube was filled with only 8ml of porc fat (FF=100%). To do this, porc fat was liquified into an oven at 100°C and mixed with agar solutions in order to obtain 1.5% final gels. The mixture was emulsified by hand during 30s for 100% and 50%, or 50s for 25% in order to obtain homogeneous solutions. The tubes were rapidly placed into liquid nitrogen to freeze the preparations. Finally, 1ml of water was placed into the surface of the gels and potential bubbles were removed with a needle. A 3D printed bubble trap was placed on top in order to avoid large bubbles and to reduce the interface volume.The 8 tubes were positioned in a commercially available phantom (MultiSample120, Gold Standard Phantoms, United Kingdom) filled with tap water (Figure 2).
MRI protocol
Acquisitions were carried out on a Siemens 3T PRISMA scanner, using the 64-channel head coil. For T1 mapping, a MP2RAGE-CS4 sequence was used2 with 1mm isotropic spatial resolution; echo train length =125; TE/TR= 3.5/7 ms; α1/α2= 7°/7°; TI1/TI2/MP2RAGE_TR, 800/2200/5000 ms; acquisition time = 3min30s; For T2 mapping, a DESS sequence was used with 1.2 mm isotropic spatial resolution; TE/TR= 5/15ms, angle: 10°; acquisition time = 6min15s.
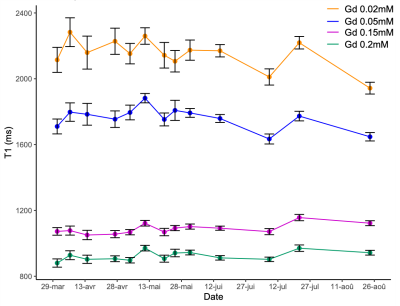
For FF, a DIXON sequence was used: 1.3mm in plane and 1.5mm thickness; TE1/TE2/TR = 1.34/2.57/4 ms; α= 9°; acquisition time =21s. The phantom was scanned using this protocol once every 1-2 weeks, during 6 months.
Analysis
Circular region-of-interest (ROI) were drawn on the parametric maps using Matlab either at the bottom of the tubes, or at the interface with the bubble trap. Mean T1, T2 and FF inside the ROI and the corresponding standard deviation were measured.
RESULTS
Typical T1 parametric map was shown on Figure 2.B.The presence of the bubble trap allows to quickly obtain stable solutions and thus constant T1 and T2 values in the Gd-DOTA vials. The concentrations of Gd-DOTA used here enabled to cover a wide range of T1 (2146ms (mimicking cerebrospinal fluid); 1776ms; 1178 ms; 994ms (mimicking white matter) but a small range of T2 values (from 72 to 82ms). The stability of the phantom was evaluated over time (6 months) and shows very weak standard deviation (Figure 3).
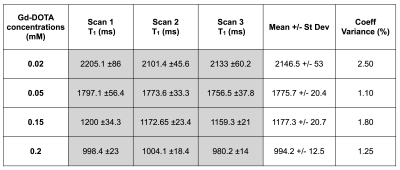
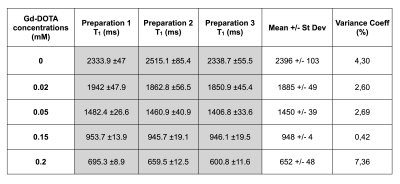
The phantom was scanned 3 times after repositioning and showed a high reproducibility of the T1 measurements (coefficients of variance of less than 3% for all the tubes) (Figure 4). Also, the phantom was made 3 times independently, and showed a very high accuracy in the protocol, as the coefficients of variance of the T1 measurements were less than 5% for all the tubes (Figure 5).
The tubes containing porc fat enabled to enlarge the Gd-DOTA relaxation times range with short T1 (304ms; 1020ms; 3003ms) and short T2 (18ms; 23; 28ms) values. Also, MR spectroscopy confirmed the amount of fat per tube. The FF within this tubes, measured by the DIXON sequence, was 96.5%, 76.8% and 62.2%, respectively.
DISCUSSION
The preparation protocol enabled to obtain a highly reproducible and stable phantom. The different concentrations of Gd-DOTA can be easily adapted by the users to mimic specific tissue. Due to the porc fat, a large range of T1 and T2 can be measured, as well as FF. The stability of the phantom should be studied for a period longer than one year.This phantom will enable to develop a more accurate MR method to measure FF than the DIXON method3.
CONCLUSION
This phantom is easy and fast to make, cheap, does not necessitate expensive and hard-to-find materials, and employs animal fat to get closer to human adipose tissue. This phantom can be used for quality control of parametric mapping sequence, but also to validate new methods. A video showing the different stages of preparation will be made available to the community.Acknowledgements
This work was supported by The French National Research Agency (ANR-19-CE19-0014) and the pIBIO imaging facility (https://pibio-bordeaux.cnrs.fr/).References
[1] Bush EC, Gifford A, Coolbaugh CL, et al. Fat-Water Phantoms for Magnetic Resonance Imaging Validation: A Flexible and Scalable Protocol. J. Vis. Exp. 2018;(139) e57704.
[2] Trotier AJ, Dilharreguy B, Anandra S, et al. The Compressed Sensing MP2RAGE as a Surrogate to the MPRAGE for Neuroimaging at 3 T. Invest Radiol. 2022; 57(6):366-378.
[3] Hayashi T, Saitoh S, Takahashi J et al. Hepatic fat quantification using the two-point Dixon method and fat color maps based on non-alcoholic fatty liver disease activity score. Hepatology Res. 2017; 47: 455–464
Figures

Figure 1 : Gd-DOTA and fat phantom preparation.
A) Aqueous solution of Gd-DOTA were mixed with 3% agarose gel and homogenized to obtain 0.02 to 0.2 mM Gd-DOTA and 1.5% Agarose gel
B) Commercial porc fat and agarose gel were mixed and emulsified to obtain solutions at 100%, 50% and 25% fat.
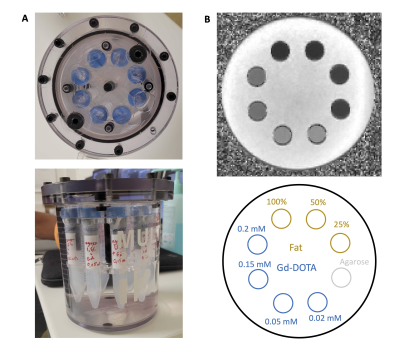
Figure 2 : MRI phantom.
A) Gd-DOTA and Fat tubes were inserted into a MultiSample120 Gold Standard Phantoms, United Kingdom filled with tap water.
B) Example of a MRI image obtained with the multisample phantom