5227
UV-Curable Hydrogels for Quantitative T1/T2 in Slice Phantoms
Szu Ting Tung1, Jolene Huey1, Michael Lustig 1, Ana Claudia Arias1, and Anita Flynn1
1Department of Electrical Engineering and Computer Science, University of California, Berkeley, Berkeley, CA, United States
1Department of Electrical Engineering and Computer Science, University of California, Berkeley, Berkeley, CA, United States
Synopsis
Keywords: Phantoms, Multi-Contrast
We developed a high-volume, low error UV-curable hydrogel formation process that can target specific T1 and T2 parameters. Our process relies on the polymerization of water-soluble photoinitiator TPO-Lithium in acrylamide (monomer), polyethylene-glycol-diacrylate (crosslinker), de-ionized (DI) water, and paramagnetic ions. We characterize the linearizing effect of varying paramagnetic ion concentrations in determining T1 and T2 values, and present an application to construct a quantitative anatomy-mimicking brain slice phantom containing UV gels.Introduction
There is a clinical need for quantitative measurements of MRI parameters in diagnostics1. Reference phantoms containing aqueous solutions with calibrated parameters exist but lack properties like partial volume and structure that exist in-vivo. Current anthropomorphic phantoms aim to provide structure, but miss quantification and detailed anatomy2. To shift toward more quantitative anatomy-mimicking solutions, we developed slice phantoms made of doped agar gels3. However, the agar gel process has several shortcomings; heating and cooling is sensitive to evaporation and changes in concentrations, resulting in variability. Managing hot, viscous fluids often leads to air bubble formation that results in susceptibility artifacts. Finally, it is difficult to achieve very long T2s (>200ms) to mimic cerebrospinal fluid (CSF) and blood4.In this work, we propose a process for constructing and altering formulations of UV-curable hydrogels to achieve desired quantitative parameters. Our solutions containing acrylamide monomer, polyethylene glycol diacrylate crosslinker (PEGDA), and water-soluble TPO-Li (Diphenyl(2,4,6-trimethylbenzoyl)phosphine oxide) photoinitiator5 are formed upon UV-exposure (Figure 2). We achieved a broad range of T1/T2 parameters by varying quantities of acrylamide, nickel chloride (NiCl2), manganese chloride (MnCl2), and demonstrate filling a stereolithography (SLA) 3D-printed slice phantom with anatomy-mimicking parameters. The phantom was derived from the brainweb6, designed in Rhino, optimized to avoid warping, and scaled down to 5" x 4.5" to fit in a Formlabs Form 3 printer (Figure 5a). By printing individual compartments representing white-matter, gray-matter, and CSF, we can control curing.
Methods
To map T1/T2 parameters of non-doped UV gels, we prepared large stock solutions and combined appropriate amounts to make 5mL solutions in aluminum-wrapped amber vials containing 0.007g TPO-Li, 0.0035g PEGDA, and linearly-varied concentrations of acrylamide from 0.15 to 0.6g/mL (Figure 3). To characterize T1/T2 parameters of paramagnetic ions, initial gel solutions were combined with linearly-varying concentrations of NiCl2 or MnCl2 in water (Figure 4c). 4mL of each sample were pipetted into 38mm diameter petri dishes (Figure 1). Samples were cured for 60 seconds in a 395nm UV-LED curing station operating at 101W/cm2 (Figure 1c-d), and sealed with parafilm to prevent evaporation.Cured gels were scanned and mapped on a 3T GE MR750W scanner (Wakasha, Wisconsin) using inversion recovery T1 Spin-echo, and T2 Spin-echo sequences (TI: 50, 100, 150, 200, 250, 500, 750, 1000, 1250, 1500, 1750, 2000, 2250, 2500 ms; TE: 15, 25, 35, 55, 75, 95, 115, 135, 155, 175, 200, 250, 300, 350, 400 ms). Stickiness and compressibility were measured on a relative scale of 1-5 (maximum 5) by how much the gel adhered to a latex glove and could be mechanically compressed (Figure 3b-c). Stretch ratio (length of gel before breaking divided by length before stretching) was obtained through videos of stretching gels over a cm ruler (Figure 3d).
With scan data, R1=1/T1 and R2=1/T2 were estimated7 and fit linearly with dopant concentrations. Using the fit to find targets, a 3D-printed brain slice was fabricated with compartments representing different tissues, then each chamber was filled and cured (Figure 5b). The slice phantom weighted scan was imaged using 3D T1 BRAVO (TI: 225 ms) and T2 Spin-echo sequences (TE: 15ms).
Results
Figure 3 depicts an association between increased concentration of acrylamide and increased R1/R2 values, and between acrylamide concentration and gel properties. Figure 4 shows the (T1, T2) maps and linear fits to R1 and R2 derived from the data. The sample and mapping images (Figure 4a-b) are a subsample representation of the 24 doped samples created for the mapping. The scan results of the SLA-printed slice phantom constructed with doped UV gels hit target (T1, T2) tissue values of (668, 70) ms for white, (949, 102) ms for gray matter, and (1881, 1133) ms for CSF, as depicted in Figure 5.Discussion
The (T1, T2) quantitative map of acrylamide in Figure 3a shows sharp increases in R1/R2 between 0.4 and 0.45g/mL. Observations of physical gel properties show a large increase in stretch ratio and an increase in stickiness around this concentration. The sharp increase in R1/R2 may be due to a structural state change of the gel between 0.4 and 0.45g/mL.Success with T1/T2 targets in doped gels was found with 0.1g/mL acrylamide concentration. While we can achieve the T2s of white- and gray-matter via doping, the resulting T1s are shorter than those in tissue. More investigation is needed to modulate T2 while keeping T1 long.
The ability to precisely achieve target tissue parameters depends on repeatability of the gel fabrication process. Large stock solutions decrease error and achieve uniformly cured gels, which was previously difficult to achieve with heat-initiated agar gels. Additionally, the T2 of non-doped UV gels (>1000ms) is an order of magnitude longer than that of non-doped agar gels, allowing mimicking of CSF and blood2. Future work includes developing an automated procedure to precisely dispense the gel solution, aiming to achieve boundaryless slice phantoms.
Conclusion
To the best of our knowledge, this is the first demonstration of fabricating MRI phantoms using UV-curable hydrogels. We demonstrated that we are capable of using a high-volume process of combining solutions to create UV-curable gels that can achieve desired quantitative T1/T2 parameters.Acknowledgements
Authors ST and JH contributed equally. We thank the UC Berkeley Brain Imaging Center for the use of their facilities and our funding source: Siemens Medical Solutions USA, Inc..References
- Keenan, K. E., Ainslie, M., Barker, A. J., Boss, M. A., Cecil, K. M., Charles, C., ... & Zheng, J. (2018). Quantitative magnetic resonance imaging phantoms: a review and the need for a system phantom. Magnetic resonance in medicine, 79(1), 48-61.
- A.A. Martinos Center / Wald group anthropomorphic phantom builder's Wiki. https://phantoms.martinos.org/Main_Page
- Gopalan, K., Tamir, J. I., Arias, A. C., & Lustig, M. (2021). Quantitative anatomy mimicking slice phantoms. Magnetic resonance in medicine, 86(2), 1159-1166.
- Hattori, K., Ikemoto, Y., Takao, W., Ohno, S., Harimoto, T., Kanazawa, S., ... & Kato, H. (2013). Development of MRI phantom equivalent to human tissues for 3.0-T MRI. Medical physics, 40(3), 032303.
- Benedikt, S., Wang, J., Markovic, M., Moszner, N., Dietliker, K., Ovsianikov, A., ... & Liska, R. (2016). Highly efficient water-soluble visible light photoinitiators. Journal of Polymer Science Part A: Polymer Chemistry, 54(4), 473-479.
- BrainWeb: Simulated Brain Database. https://brainweb.bic.mni.mcgill.ca
- Barral, J. K., Gudmundson, E., Stikov, N., Etezadi-Amoli, M., Stoica, P., & Nishimura, D. G. (2010). A robust methodology for in vivo T1 mapping. Magnetic resonance in medicine, 64(4), 1057-1067.
Figures
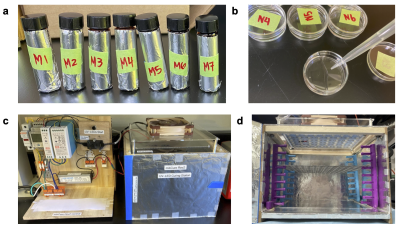
Figure 1: (A) Solutes obtained from large-volume stock solutions and mixed into 5 mL gel solutions stored in aluminum-wrapped amber vials. (B) Solutions are mixed on a vortex mixer and then pipetted into petri dishes. (c) Controller and UV-LED curing chamber (operates at 395 nm) that petri dishes are placed in. (D) Removable rack within UV-LED curing chamber for adjusting distance to control irradiance power.
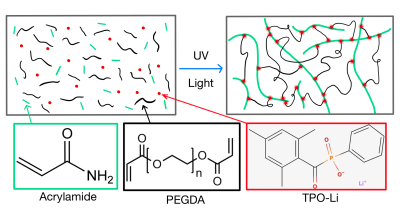
Figure 2: Illustration of UV-gel formation by way of acrylamide monomers forming polymers through TPO-Li photoinitiation and being cross-linked together by PEGDA.
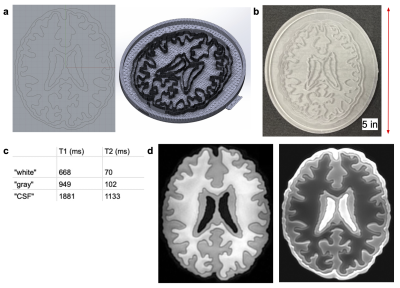
Figure 3: (A) R1 and R2 mapped values that correspond to acrylamide concentrations of 0.15 to 0.6 g/mL with fixed concentrations of TPO-Li and PEGDA. (B-D) Stickiness and compressibility (on a scale of 1-5) as well as stretch ratio (length after stretch divided by gel diameter before stretch) in terms of acrylamide concentration in g/mL.
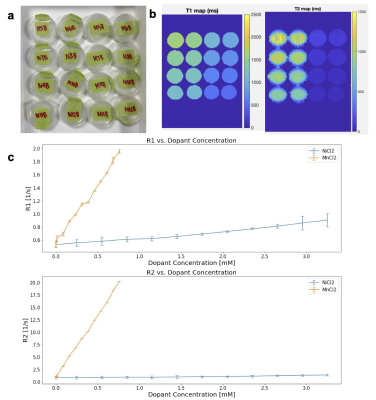
Figure 4: (A) Photo of NiCl2 and MnCl2-doped samples in scanning orientation (coronal view). (B) T1 and T2 maps of NiCl2 and MnCl2-doped samples estimated from spin echo imaging. (C) Linear relationship between gel concentration of NiCl2 and MnCl2 with R1=1/T1 and R2=1/T2 estimations.
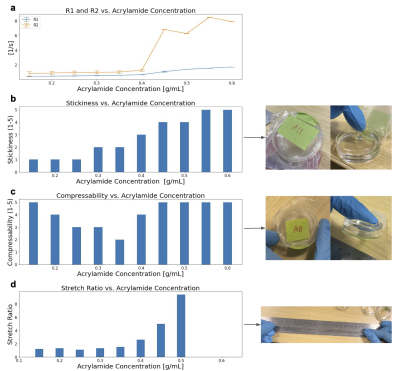
Figure 5: (A) CAD file of slice phantom created in Rhino and viewed in SolidWorks. (B) SLA-printed slice phantom with outer walls 5.5 mm tall above the 1 mm thick base; inner walls 5 mm tall; 500 µm thick outer membrane pushed outwards by 1.5 mm. (C) Table depicting T1 and T2 target values representing white-matter, gray-matter, and CSF. (D) 3D T1-weighted BRAVO scan TI=225 (left) and 2D T2-weighted spin echo scan TE=15 (right), illustrating how differently doped gels mimic target relaxation parameters.
DOI: https://doi.org/10.58530/2023/5227