5223
Design and Validation of a Multi-Modality Lung Phantom1Biomedical Engineering, Texas A&M University, College Station, TX, United States, 2Biomedical Engineering and Electrical Engineering, Texas A&M University, College Station, TX, United States
Synopsis
Keywords: Phantoms, Multimodal
Phantoms often mimic only one specific property of the body, and without relevant anatomical structure. We report the design and construction of an anatomic phantom that accurately mimics the in vivo properties of the lungs for both ultrasound and magnetic resonance imaging. The phantom demonstrated qualitative likeness to the lungs under ultrasound and quantitatively similar tissue relaxation characteristics using magnetic resonance imaging. Future work will determine if the phantom can be used to validate early-stage medical devices that operate within the lungs.Introduction
Accurate models of the human body, known as phantoms, are essential to the medical field for testing medical devices, emulating clinical imaging procedures, and for training physicians, medical students, and other healthcare professionals. Phantoms that have been reported in literature, specifically lung phantoms, are usually designed exclusively for ultrasound (US) using low-cost materials to simulate the reflective interfaces between tissues1. However, in order to have utility for multiple imaging modalities with varying intents (e.g. device testing), a phantom needs to replicate not only tissue properties but also accurate anatomy. The work presented here describes the development of a multi-modality lung phantom with a long shelf-life that accurately mimics the in vivo characteristics of the human lungs for US and magnetic resonance imaging (MRI).Materials and Methods
The lung phantom was constructed after validating the tissue mimicking materials (TMMs) accurately replicated the in vivo lung tissues under US and MRI. Furthermore, the silicone TMMs are stable for months or years and durable as compared to common phantom materials like gelatin2.All ultrasound imaging was performed using a L14-5/38 Linear Array Transducer on a Sonix Touch system (Ultrasonix). T1 and T2 measurements of various samples of potential TMMs were acquired on a 4.7T Varian Inova system before construction of the full size phantom. Reported T1 and T2 relaxation values at 3T were extrapolated to 4.7T for comparison of the muscle/fat TMM3,4. MR imaging of the fully constructed phantom was performed on a Siemens MAGNETOM Verio 3T clinical system with PD, T1, and T2 weighted multi-slice SE sequences.
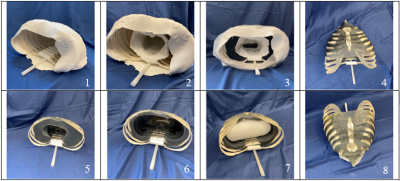
To construct the full phantom, the ribcage was first removed from a full scale PVC skeleton (Life Sized Human Skeleton Model, ZENY) and the ferrous spine was removed from the posterior side of the ribs. PVC cement (Clear, Oatey Co.) and plastic adhesive (E6000, Eclectic Products) were used in conjunction with ¼” PVC pipe to act as the connections between the back of the ribs and a ¼” nylon rod acting as the spine. This ensured the MR compatibility of the phantom. Epoxy (Clear Epoxy, Gorilla Glue) was evenly distributed around each bone to increase the acoustic backscattering in US to more closely resemble in vivo bone. A mold was formed around the ribcage with clay (Air-Dry Clay, Polyform Products Co.) so that the muscle/fat analog TMM could eventually adhere to the bones. Another clay cylinder was inserted within the ribcage to create a cavity where the lung material would eventually be placed. Three kilograms of silicone (Ecoflex 00-10, Smooth-On) mixed with 3.6% scattering agent (Graphite Powder, Asbury Carbons) was poured into the mold. This acted as the muscle/fat TMM and adhered to the ribcage once cured. The inner and outer molds were removed and a scalpel was used to hollow out the lung cavity to further match the anatomical distance between 2 cm and 5 cm from the skin surface to the pleural lining of the lung5. The lung silicone foam (Soma Foama 25, Smooth-On) was dispensed within the inner cavity and allowed to cure, acting as the lung TMM. The skin was replicated by another silicone mixture (Ecoflex 00-20, Smooth-On) which was poured over the phantom and left to cure, creating a thin layer of skin-like material. The fabrication process is pictorially illustrated in Figure 1.
Results and Discussion
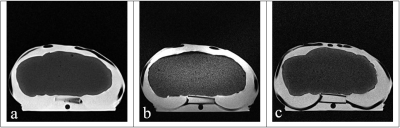
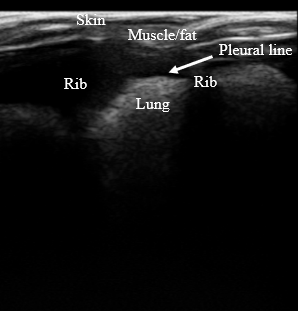
The design of the lung phantom was validated by accurately mimicking the anatomy of in vivo lungs (Figure 2), replicating the visual appearance of the lungs in US (Figure 3), and maintaining accurate relaxation values in MRI to reported tissue values. A summary of the TMMs used and relaxation parameters are shown in Table 1. Comparison between the muscle/fat material and in vivo muscle/fat tissue demonstrated a 2% difference in T1 relaxation and a 19% difference in the T2 relaxation at 4.7T.Conclusion
Qualitative US and quantitative MRI analysis verified the proposed lung phantom design for accurate modeling of the human lungs. A future study will be conducted to determine if the lung phantom can be a viable testing platform for early-stage medical devices.Acknowledgements
The authors gratefully acknowledge the funding for this project through the NIH RO1 CA254964. The authors also gratefully acknowledge Dr. Raffaella Righetti for assistance with the US imaging studies.
References
[1] H. H. Do and S. Lee, "A Low-Cost Training Phantom for Lung Ultrasonography," Chest, vol. 150, no. 6, pp. 1417-1419, 2016, doi: 10.1016/j.chest.2016.09.033.
[2] K. Zell, J. I. Sperl, M. W. Vogel, R. Niessner, and C. Haisch, "Acoustical properties of selected tissue phantom materials for ultrasound imaging," Physics in Medicine and Biology, vol. 52, no. 20, pp. N475-N484, 2007, doi: 10.1088/0031-9155/52/20/n02.
[3] J. Z. Bojorquez, S. Bricq, C. Acquitter, F. Brunotte, P. M. Walker, and A. Lalande, "What are normal relaxation times of tissues at 3 T?," (in eng), Magn Reson Imaging, vol. 35, pp. 69-80, Jan 2017, doi: 10.1016/j.mri.2016.08.021.
[4] P. A. Bottomley, T. H. Foster, R. E. Argersinger, and L. M. Pfeifer, "A review of normal tissue hydrogen NMR relaxation times and relaxation mechanisms from 1-100 MHz: dependence on tissue type, NMR frequency, temperature, species, excision, and age," Med Phys, vol. 11, no. 4, pp. 425-48, Jul-Aug 1984, doi: 10.1118/1.595535.
[5] K. Inaba et al., "Radiologic Evaluation of Alternative Sites for Needle Decompression of Tension Pneumothorax," Archives of Surgery, vol. 147, no. 9, 2012, doi: 10.1001/archsurg.2012.751.