5221
Realistic human head phantoms for 7T sequence development, parallel transmission & spectroscopy methodology1German Center for Neurodegenerative Diseases (DZNE), Bonn, Germany, 2Physics and Astronomy, University of Bonn, Bonn, Germany
Synopsis
Keywords: Phantoms, RF Pulse Design & Fields, Spectroscopy
An optimized human head phantom design for 7 Tesla field strength and its standardized fabrication process is presented. Two different phantom versions were produced: The first gel-only version can be set up quickly and provided realistic T1 and T2 values for its brain, muscle, and lipid compartments. The second version, with its liquid brain compartment containing several metabolites, can be utilized for spectroscopy sequence development. With the 3D model and the validated recipes mimicking head and brain tissue, extensions to other applications can be more easily achieved, such as system characterization, diffusion weighted imaging, or realistic flow phantoms.
Introduction
This work’s goal was the standardized construction of a phantom that mimics the most relevant physical properties of human brain, muscle, lipid, and skull with air-filled cavities at 7T. A realistic spectroscopy model at single-channel transmit (Tx) 7T (1) was the basis for an improved fabrication process, which can be easily tailored to specific research focuses. Additionally, the phantom is especially designed for parallel transmission (pTx) applications.Methods
Based on the publicly available adult male 3D-model (2) two versions with 95% of the original size and three separate fillable compartments were designed, printed in nylon (PA12) and were glued waterproof (Figure 1). For both phantom versions the respective brain and muscle compartment’s base recipes, containing purified water (H2O), sodium chloride (NaCl), polyvinylpyrrolidon (PVP K30), and Agarose, were conducted on Mediaclave-10/30 (Integra Bioscience AG, Zizers, Switzerland): With continuous stirring, the homogeneous suspension was heated to 80°C for one hour, sterilized by 100°C for five minutes, bottled at 70°C, and was gradually cooled down in a thermo cabinet.For the first version, named Head-Gel, the 1.1L brain compartment was made of 78.1% H2O, 19.0% PVP K30, 1.5% NaCl, and 1.4% Agarose for a target conductivity σ = 0.55S/m and relative permittivity εr = 51.98 (3). The 1.1L muscle gel consists of 77.4% H2O, 19.0% PVP K30, 1.5% NaCl, and 2.1% Agarose for σ = 0.77S/m, εr = 58.24 (3). For the 0.4L lipid compartment butter ghee, containing 99.8% cow’s milk fat with σ = 0.04S/m, εr = 5.64 (3), was used.
For the second version, named Head-Spectro, the muscle and fat compartments were filled with the previous described ingredients, but here the 1.1L liquid brain consists of 60.06% H2O, 38.63% PVP K30, and 0.96% NaCl in which after cooling down to room temperature the following substances were dissolved: 0.13% N-Acetyl-L-aspartic acid (NAA), 0.1% L-Glutamic acid (Cho), 0.06% Creatine (Cr), 0.05% myo-Inositol, and 0.01% y-Aminobutyric acid (GABA). Remaining suspensions were filled into 0.5L glass bottles with 8cm diameter each.
All experiments were conducted on a 7T Plus Magnetom scanner (Siemens Healthineers AG, Erlangen, Germany) with a 1Tx/32-channel receive (Rx) and 8Tx/32Rx head coil (Nova Medical Inc, Wilmington, USA) together with a shoulder phantom (4). After a minimum one-week-storage at 20°C ambient MRI room temperature, relaxometry measurements (5) for each suspension, by two-point T1-IR and T2-SE with two-point fit, were carried out on consecutive days with the 0.5L glas bottle centred within the 1Tx/32Rx head coil.
Likewise for both phantoms and in vivo, were B0 maps acquired by a vendor provided 2-echo GRE, (3mm)3; matrix=78x78x60; TE1,2/TR=0.75ms, 1.94ms/31.3ms; FA=8°, and the single-channel B1+ mapped with AFI (6), (4mm)3; matrix=56x56x44; TE/TR=1.12ms/20ms; FA=85°, for the 8Tx/32Rx head coil. A circular polarized (CP) mode MPRAGE sequence was acquired for Head-Spectro with parameters (0.6mm)3; matrix=428x364x256; TE/TR/TI=2.06ms/2.5s/1.1s; FA=5°; water excitation. Additionally, was an universal pulse (UP) MPRAGE, from the PASTeUR package (7) with the prior parameters conducted, in combination with an AFI B1+ map, (4mm)3; matrix=56x56x44; TE/TR=2.04ms/20ms; FAnominal=20°. Spectra were acquired using a custom MEGA-sLASER sequence (8). The editing frequency was set to 1.7ppm; TE/TR=5ms/72ms; 32 excitations.
Results
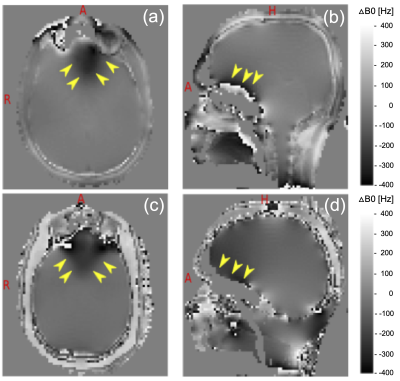
The relaxometry result in least-square fitted mean T1muscle=1486ms; T2muscle=34ms; T1fat=397ms; T2fat=40ms. Respectively, for Head-Gel T1brain-gel=1600ms; T2brain-gel=52ms and for Head-Spectro T1brain-liquid=1012ms; T2brain-liquid=513ms was distinguished.The B0 results (Figure 2), show good agreement of the susceptibility artifact prone orbito-frontal brain region within Head-Gel in contrast to the in vivo situation.
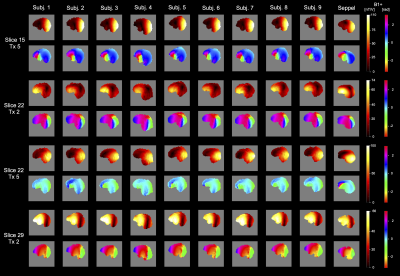
A comparison between Head-Spectro’s single-channel B1+ and 9 subjects (4 female, 5 male) is depicted in Figure 3. The maps show good similarity of the transmit field distributions. The standard CP-mode of the 1Tx/32Rx head coil can be recognized qualitatively in Figure 4(a). Additionally, the water selection efficiency to suppress the subcutaneous fat layer mimicking compartment signal within Head-Spectro is demonstrated. Moreover, the calibration-free UP’s (9) provide excellent transmit field homogenization for Head-Spectro (Figure 4(b)+(c)).
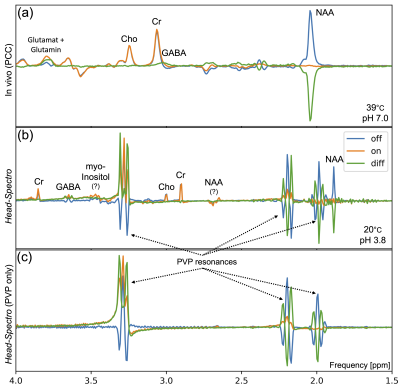
The single voxel spectroscopy in the central area of Head-Spectro was compared with the posterior cingulate cortex (PCC) result of an exemplary subject (8). Figure 5 shows the MEGA-sLASER spectra’s real part. The largest visible resonances are the PVP multiplets, which were already present before adding the spectroscopy ingredients. Otherwise, the spectra show expected features: Due to the non-physiological pH-value (10) and temperature (11), four of these resonances are shifted by approximately -0.2ppm and GABA by +0.6ppm, compared to their in vivo counterparts. The different resonance shapes (edit-off: anti-phase triplet, edit-on: triplet) indicate effective J-editing.
Discussion & Conclusion
We present a head phantom design which can be tailored to different research questions at 7T. The design was realized for the fabrication of two phantoms, Head-Gel and Head-Spectro. For single voxel spectroscopy the PVP resonances dominate. However, neither the signals of the main metabolites, nor the J-edited GABA overlap with those resonances. Therefore, Head-Spectro is well suited for spectroscopic methodology. The applicability of Head-Gel for T1-, T2-mapping and diffusion weighted imaging (12) will be further investigated, while the usefulness of both presented phantoms for pTx techniques, like binomial kT-points (13), extends to a point where it makes more sense to do further development in vivo. Since the UP calculation is based on human data, there will always be a gap between reality and phantoms.Acknowledgements
The authors thank Thomas Pazulla (TP Technische Dienstleistungen, Oderwitz, Germany) for his valuable help on the 3D model construction. This work received financial support from the German Federal Ministry of Education and Research (BMBF; funding code 01ED2109A) as part of the SCAIFIELD project under the aegis of the EU Joint Programme - Neurodegenerative Disease Research (JPND) (www.jpnd.eu).References
(1) Jona G, Furman-Haran E, Schmidt R. Realistic head-shaped phantom with brain-mimicking metabolites for 7T spectroscopy and spectroscopic imaging. NMR in Biomedicine 2021;34:e4421.
(2) Guérin B, Stockmann JP, Baboli M, Torrado-Carvajal A, Stenger AV, Wald LL. Robust time-shifted spoke pulse design in the presence of large B0 variations with simultaneous reduction of through-plane dephasing, B1+ effects, and the specific absorption rate using parallel transmission. Magnetic Resonance in Medicine 2016;76:540–554.
(3) Wood S, Santini T, Krishnamurthy N, Martins T, Farhat N, Ibrahim TS. A comprehensive electromagnetic evaluation of an MRI anthropomorphic head phantom. NMR in Biomedicine 2021;34:e4441.
(4) Voelker MN, Kraff O, Pracht E, Wollrab A, Bitz AK, Stöcker T, Quick HH, Speck O, Ladd ME. Quality assurance phantoms and procedures for UHF MRI—the german ultrahigh field imaging (GUFI) approach. In: Proceedings of the 25th annual meeting of ISMRM, Honolulu, USA. Vol. 3912.; 2017.
(5) Carneiro AAO, Vilela G, De Araujo D, Baffa O. MRI relaxometry: Methods and applications. Brazilian Journal of Physics 2006;36:9-15.
(6) Yarnykh VL. Actual flip-angle imaging in the pulsed steady state: A method for rapid three-dimensional mapping of the transmitted radiofrequency field. Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine 2007;57:192–200.
(7) Massire A, Mauconduit F, Gras V, Lapert M, Naudin M, Guillevin R, Vignaud A, Boulant N. PASTEUR: Package of anatomical sequences using parallel transmission universal pulses now available for MAGNETOM terra.
(8) Völzke Y, Pracht ED, Hattingen E, Y Tse DH, Stöcker T. On the reproducibility of hippocampal MEGA-sLASER GABA MRS at 7T using an optimized analysis pipeline. Magnetic Resonance Materials in Physics, Biology and Medicine 2021;34:427–436.
(9) Gras V, Vignaud A, Amadon A, Le Bihan D, Boulant N. Universal pulses: A new concept for calibration-free parallel transmission. Magnetic Resonance in Medicine 2017;77:635–643.
(10) Korzowski A, Weinfurtner N, Mueller S, Breitling J, Goerke S, Schlemmer H-P, Ladd ME, Paech D, Bachert P. Volumetric mapping of intra-and extracellular pH in the human brain using 31P MRSI at 7T. Magnetic resonance in medicine 2020;84:1707–1723.
(11) Rzechorzek NM, Thrippleton MJ, Chappell FM, et al. A daily temperature rhythm in the human brain predicts survival after brain injury. Brain 2022;145:2031–2048.
(12) Wagner F, Laun FB, Kuder TA, et al. Temperature and concentration calibration of aqueous polyvinylpyrrolidone (PVP) solutions for isotropic diffusion MRI phantoms. PloS one 2017;12:e0179276.
(13) Löwen D, Pracht ED, Stirnberg R, Liebig P, Stöcker T. Interleaved binomial kT-points for water-selective imaging at 7T. Magnetic Resonance in Medicine 2022.
Figures
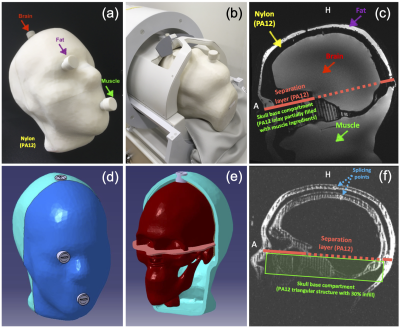
Figure 1. (a)+(b) First phantom version Head-Gel with elevated filling nozzles for the three different solid substance compartments and position within the 1Tx/32Rx head coil. (c) Respective four compartment and separation layer location on a sagittal slice. (d)+(e) Second phantom 3D-model Head-Spectro, depicting the recessed fillers and outer separation layer position. (f) Short echo GRE sagittal slice of Head-Gel prior to the filling process, showing the nylon structure and splicing between the two inner skull/brain shells and the two outer skin shells.