5218
Epileptogenic periventricular nodular heterotopia BOLD fluctuations are anti-correlated to non-epileptogenic nodular heterotopias1Imaging Institute, Cleveland Clinic, Cleveland, OH, United States, 2Neurologic Institute, Cleveland Clinic, Cleveland, OH, United States
Synopsis
Keywords: Epilepsy, fMRI (resting state)
In this study, using a limited sample size, we establish with 95% confidence that epileptogenic and non-epileptogenic periventricular nodular heterotopias can be distinguished based on their relative resting state connectivityIntroduction
Periventricular nodular heterotopias (PVNHs, or nodules) account for 15-20% of all malformations of cortical development, and are frequently associated with pharmacoresistant epilepsy. While surgical resection or ablation of the epileptogenic zone is the most effective intervention to achieve seizure freedom in epilepsy patients, localizing the epileptogenic zone in epilepsies associated with PVNH is currently one of the greatest clinical challenges for epilepsy centers worldwide. Although invasive evaluation with stereotactic-electroencephalography (SEEG) is usually a necessary step for the localization of epileptogenic zone in patients with PVNH, SEEG electrodes cannot fully sample the often numerous and frequently bilateral nodules for practical reasons. In this study, we investigated differences between rsfMRI properties of epileptogenic nodules and non-epileptogenic nodules in the same patient. Although the study sample was small, we determine with 95% confidence that epileptogenic nodules are inversely correlated to the spontaneous BOLD fluctuations in non-epileptogenic nodules, potentially providing clues to noninvasively separate them.Methods
Image Acquisition:
Six subjects with PVNH nodules (identified from prior clinical imaging) and medically refractory epilepsy were scanned in an IRB-approved protocol to measure rsfMRI connectivity. Scans in included 1) High resolution T1-weighted anatomic scan (MPRAGE: 192 0.94mm thick axial slices, voxel size = 0. 8 x 0.8 x 1mm3, TR/TI/TE = 1900 ms, 800ms, TE = 2.57 ms, FOV = 24 cm x 24 cm, flip angle = 10°), 2) resting state fMRI scan (2D GRE EPI, 39-3mm thick axial, 180 volumes, TR = 2.170s, TE = 30 ms, voxel size = 3 x 3 x 3mm3, FOV = 24 cm x 24 cm, flip angle = 80°).
Identification of epileptogenic nodules:
SEEG was performed to localize the epileptogenic zone as part of the patients’ standard of care. SEEG electrode reconstruction was performed by fusing the postoperative thin-sliced CT images with the MPRAGE images using automatic full-volume maximal-mutual-information registration in Curry 8 (Compumedics NeuroScan, Australia). Classification of seizure-onset zone (SOZ) and SEEG electrode reconstruction was performed by fusing the postoperative thin-sliced CT images with the MPRAGE images using automatic full-volume maximal-mutual-information registration in Curry 8 (Compumedics NeuroScan, Australia). Classification of seizure-onset zone (SOZ) and non-SOZ SEEG contacts were obtained from the official clinical report, based on consensus at the epilepsy patient management conference (PMC). If the nodules were implanted by SEEG and included in the SOZ (per official clinical report), they were considered epileptic nodules. If nodules were implanted by SEEG and shown to be not involved at SOZ, they were considered non-epileptic nodules. If the nodules were not implanted by SEEG (per PMC consensus based on noninvasive evaluation data), they were also considered as non-epileptic nodules; this was a fair assumption given the patients included in this cohort had sustained seizure freedom or significant seizure reduction after the SEEG-directed surgery which did not involve these nodules. Masks of epileptic/ non-epileptic nodules were manually created on the clinical T1w MPRAGE images using MRIcron. Masks of the cortical SOZ electrode contacts were created based on the CT image coregistered with the same MPRAGE images that nodule masks were created.
Image Analysis:
rsfMRI Postprocessing:
All rsfMRI data were processed in the following manner:
1. Physiologic noise removal (PESTICA1)
2. Motion correction (SLOMOCO2)
3. Temporal band-pass filtering (0.001-0.01Hz)
Nodule Connectivity Analysis:
Nodule masks were created using the MPRAGE images for each subject. Each nodule mask was identified as epileptogenic or non-epileptogenic based on the SEEG criteria described above. A single representative timecourse was produced from the rsfMRI data for each nodule mask by combining all voxels within the mask using a cohesive parcellation procedure3. Z-score maps were produced for each epileptogenic mask timecourse by calculating the Pearson cross correlation to the timeseries for all brain tissue voxels and performing the z-score procedure described in Lowe et al.4. Mean z-score to all non-epileptogenic nodule parcels was calculated.
Results
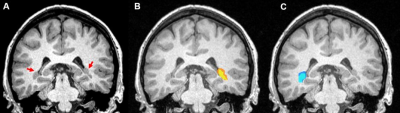
Figure 1 shows example nodule masks for a subject. Two subjects’ data were exclulded due to the lack of non-epileptogenic nodules. The remaining 4 subjects’ data epileptogenic-non-epileptogenic nodule connectivity are summarized in Table 1.The mean and standard error z-score connectivity is reported in Table 1. Based on this small sample size, we determine with 95% confidence that non-epileptogenic PVNH resting state timeseries are inversely correlated to non-epileptogenic PVNH nodules
| Study | 1 | 2 | 3 | 4 | Mean ± SE |
| Epileptic-non Epileptic Nodule z-score | -0.76 | 0.10 | -0.11 | -0.38 | -0.29 ± 0.09 |
Table 1: Z-score of rsfMRI timeseries correlation of epileptogenic nodules to non-epileptogenic nodules.
Discussion
A major challenge in clinical management of medically refractory epilepsy patients with PVNH is identifying the nodules that are candidate epileptogenic regions for surgical resection. Our finding indicates a possible non-invasive way of distinguishing epileptogenic and benign PVNH regions by the polarity of their relative correlation. Further study is necessary to confirm this result and characterize the relationship of the connectivity of the epileptogenic PVNH regions to nearby cortex to further classify the epileptogenic nature of the nodules.Conclusion
We present a study in a limited sample of epilepsy patients with PVNH nodules. We demonstrate with 95% confidence that epileptogenic and non-epileptogenic nodules have a distinct connectivity relationship that can be used to non-invasively classify nodules as epileptogenic.Acknowledgements
This study was supported by the Cleveland Clinic Neurological Institute Transformative Neuroscience Award.
References
1. E. Beall, M.J. Lowe. Isolating physiologic noise sources with independently determined spatial measures. NeuroImage, 37(4),1286-300,(2007).
2. Beall, E.B., Lowe, M.J. SimPACE: Generating simulated motion corrupted BOLD data with synthetic-navigated acquisition for the development and evaluation of SLOMOCO: A new, highly effective slicewise motion correction. Neuroimage 101C, 21-34, 2014
3. A.Nemani, M.J. Lowe, Cohesive Parcellation of the human brain using resting-state fMRI., J. Neurosci Methods. 2022 Jul 15;377:109629. doi: 10.1016/j.jneumeth.2022.109629. Epub 2022 May 23.
4. MJ Lowe, BJ Mock, JA Sorenson. Functional connectivity in single and multi-slice echoplanar imaging using resting-state fluctuations. NeuroImage, 7:119-132 (1998). PMID: 9558644