5217
Neurite orientation and dispersion density imaging (NODDI) to detect PCDH19 related microstructural anomalies.1Bambino Gesù Children's Hospital, Rome, Italy
Synopsis
Keywords: Epilepsy, Diffusion/other diffusion imaging techniques, NODDI
Protocadherin 19 (PCDH19)-clustering epilepsy (PCDH19-CE) is a genetic form of epilepsy caused by a mutation of PCDH19-CE gene that begins in the first year of life. Patients with this disorder may present intellectual disability, behavioral problems and motor and language delay. Using advanced Magnetic Resonance Imaging (MRI) methods we were able to highlight microstructural changes of brain in PCDH patients. These results shows that Neurite orientation dispersion and density imaging (NODDI) techniques might be useful tools to investigate PCDH19 anomalous arborization.Introduction
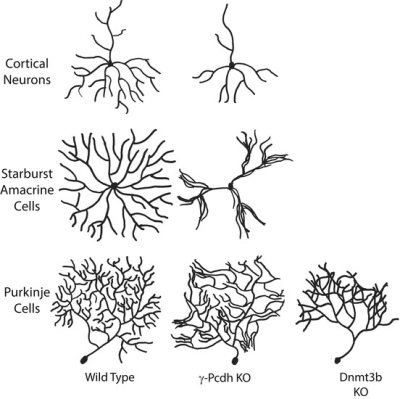
The PCDH19-CE gene is localized on the long arm of chromosome X (Xq22.3). Unlike the “classical” X-linked disorders, PCDH19-CE mainly affects females and while males generally do not present seizures or ID,. PCDH19-CE is predominantly expressed in the and temporal and limbic regions of developing and adult nervous system with important roles in neural development and function, such as neuronal differentiation, axon guidance, dendritic arborization and self-avoidance1, and synapse formation and dynamics2. Currently, the most promising technique for the quantification of neurite morphology, such as dendrite arborization, is a diffusion MRI technique known as neurite orientation dispersion and density imaging (NODDI). This technique is based on a multi-shell high-angular resolution diffusion imaging protocol and offers a novel way to analyze diffusion-weighted data and infer on tissue microstructure properties. The aim of the study was then to prove how NODDI would be able to assess in vivo dentridic arborization as previously demonstrated in animal model of PCDH gene mutation3.Methods
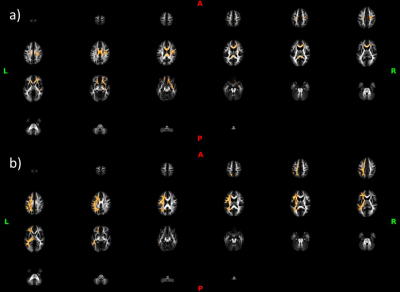
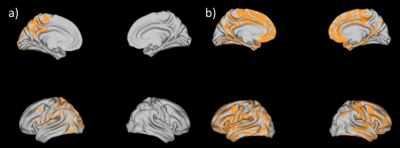
4D multishell diffusion images as well as structural ones were acquired for each of the 21 participants (8 PCDH patient and 13 controls) with a Siemens MAGNTOM Skyra 3T MRI scanner (Repetition time: 6400 ms, Voxel size:2x2x2 mm3, Number of slices: 66, Flip Angle: 90°). Data were acquired at four different b-values; (1) b = 300 s/mm2 along 10 gradient direction, (2) b = 700 s/mm2 and (3) b = 1000s/mm2, both with gradients along 30 directions and (4) b = 2000s/mm2, with 64 directions. All diffusion images were acquired with a spatial resolution of 2.0 × 2.0 × 2.0 mm and acquisition matrix of 128 × 128. The 4D multi-shell images were denoised and corrected for eddy current-induced distortion and motion artifacts. The diffusion model Neurite Orientation Dispersion Density Imaging (NODDI) and Bingham-NODDI were fitted with the GPU-based computation toolbox MDT (Microstructure Diffusion Toolbox4). Orientation dispersion index of NODDI (ODI), Isotropic volume fraction (v-iso) and intra-cellular volume fraction (v-ic) maps were derived. Each map from all subjects was aligned to a standard template by a nonlinear coregistration. The aligned maps were then averaged to produce a group mean image, and ODI skeletons were generated highlighting the tracts common to the entire groups. For each subject, a ODI threshold of 0.2 was used before projecting the aligned maps onto the skeleton. The same steps were used for v-ic and v-iso maps. Statistical analysis on white matter (WM) was then performed using FSL Randomize with Threshold-Free Cluster Enhancement (TFCE) and corrected for multiple comparisons. The NODDI maps were mapped onto the cortical surface using an algorithm weighted towards the cortical mid-thickness5 and Connectome Workbench v.1.3.2 (https://github.com/Washington-University/workbench). Statistical analysis on gray matter (GM) were obtained with PALM (from FSL with TFCE and randomization multiple comparison approach).Results
Orientation dispersion index of NODDI (ODI) resulted higher for PCDH19 patients in the WM and the GM. Isotropic volume fraction (v-iso) was higher in the WM of PCDH19 patients. Gray matter statistically intra-cellular volume fraction (v-ic) was significantly higher in control group with respect to PCDH19 patients group in GM and specifically in cingulate, superior frontal, paracentral and frontal regions.Discussion
These developmental abnormalities across WM and GM likely represent a measurable anatomic counterpart of the reduced contribution of the PCDH19-CE protein to local cortical folding. Furthermore, the increase in ODI seems to probably linked to the abnormal microsctructure seen in PCDH19 model animals. These findings point out how ODI map might be a possible biomarker of anomalous arborization and this condition might be a very specific proof of this hypothesis.Conclusion
PCDH19-CE Epilepsy is a genetic form of is caused by a mutation of PCDH19-CE gene. Neurite orientation dispersion and density imaging (NODDI) is a promising technique for the quantification of a well-specified neurite morphology. Our results confirms the potential use of this diffusion MRI technique as a biomarker of anomalous arborization, specifically in PCDH19 pathology.Acknowledgements
No acknowledgement found.References
1. Biswas S, Emond MR, Duy PQ, Hao LT, Beattie CE, Jontes JD. Protocadherin-18b interacts with Nap1 to control motor axon growth and arborization in zebrafish. Mol Biol Cell. 2014;25(5):633–42.
2. Compagnucci C, Petrini S, Higuraschi N, Trivisano M, Specchio N, Hirose S, et al. Characterizing PCDH19 in human induced pluripotent stem cells (iPSCs) and iPSC-derived developing neurons: Emerging role of a protein involved in controlling polarity during neurogenesis. Oncotarget. 2015;6(29):26804–13.
3. Mak E, Holland N, Jones PS, Savulich G, Low A, Malpetti M, et al. In vivo coupling of dendritic complexity with presynaptic density in primary tauopathies. Neurobiol Aging [Internet]. 2021;101:187–98. Available from: https://doi.org/10.1016/j.neurobiolaging.2021.01.016
4. Harms RL, Fritz FJ, Tobisch A, Goebel R, Roebroeck A. Robust and fast nonlinear optimization of diffusion MRI microstructure models. Neuroimage [Internet]. 2017;155(April):82–96. Available from: http://dx.doi.org/10.1016/j.neuroimage.2017.04.064
5. Glasser MF, van Essen DC. Mapping human cortical areas in vivo based on myelin content as revealed by T1- and T2-weighted MRI. J Neurosci. 2011;31(32):11597–616.
Figures