5216
Quantitative Comparison of 1.5T and 3.0T 1H Magnetic Resonance Spectroscopy for Temporal Lobe Epilepsy1Department of Instrumental and Electrical Engineering, Xiamen University, Xiamen, China, 2Institute of Artificial Intelligence, Xiamen University, Xiamen, China, 3Biomedical Intelligent Cloud R&D Center, Fujian Provincial Key Laboratory of Plasma and Magnetic Resonance, Department of Electronic Science, Xiamen University, Xiamen, China, 4Department of Information & Computational Mathematics, Xiamen University, Xiamen, China, 5Department of Neurology, The Second Affiliated Hospital of Xiamen Medical College, Xiamen, China, 6National Institute for Data Science in Health and Medicine, Xiamen University, Xiamen, China, 7Department of Radiology, The Second Affiliated Hospital of Xiamen Medical College, Xiamen, China
Synopsis
Keywords: Epilepsy, Brain
The study explored the diagnostic utility of different field strengths for the temporal lobe epilepsy (TLE) with 1H magnetic resonance spectroscopy (1H-MRS) which could be utilized to examine the concentrations of related metabolites. Four ratios of brain metabolites, including NAA/Cr, NAA/Cho, NAA/(Cho+Cr) and Cho/Cr were introduced to four control experiments with the Mann-Whitney U Test, the power analysis and the Paired T-Test adopted. Results suggested that 1.5T and 3.0T scanners might have comparable potential in distinguishing TLEs from HCs when 1H-MRS was used to identify patients with TLE.Purpose
Epilepsy is a kind of serious neurological disorder of the brain[1]. During this disease attack, a loss of consciousness, disturbances of limbs movement and other symptoms will occur, but the diagnosis of TLE is still challenging since some resources, including the gold standard, complete clinical history and reliable patient testimony are not accessible [1, 2]. Video electroencephalogram (V-EEG) and magnetic resonance imaging (MRI) are typical of current diagnosis techniques [2], but also have disadvantages. Studies have shown that TLE may be caused by brain damage or genetic mutations[3]. Brain metabolite concentrations can be measured by 1H-MRS, mainly including N-acetylaspartate (NAA), creatine (Cr), and choline (Cho) [4, 5]. Therefore, 1H-MRS was used and different field strengths were introduced to study the differences in the diagnostic utility of 1H-MRS for TLE at 1.5T and 3.0T.Method
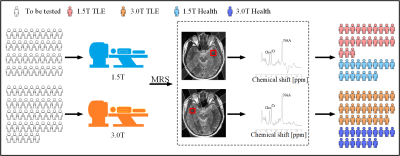
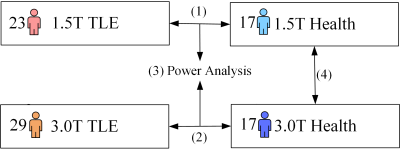
There were 23 TLE patients diagnosed with the 1.5T scanner (15 males and 8 females, age: 29.52±13[Mean±Standard Deviation (SD)]), 29 TLE patients diagnosed with the 3.0T scanner (20 males and 9 females, age: 28.2±9.0), and 17 healthy controls (HCs) (11 males and 6 females, age: 23.35±4.11) with the 1.5T scanner and 3.0T scanner, respectively. The 1H-MRS data collection process was shown in Figure 1. To minimize the impact of multi-factor experimental conditions on outcomes, data were collected using scanners with different field strengths on the same healthy volunteer. The 1H-MRS data were acquired from the bilateral temporal poles of all subjects, and four control experiments were designed (Figure 2) as followed: (1) 1.5T TLE group vs. 1.5T HCs; (2) 3.0T TLE group vs. 3.0T HCs; (3) the power analysis between the 3.0T scanner and 1.5T scanner based on the statistical test of the TLE and HCs; and (4) 3.0T HCs vs. 1.5T HCs. The comparisons of the spectrums obtained in the control experiment were shown in Figure 3. These comparisons were aimed at evaluating the differences in the diagnostic utility of TLE at 1.5T and 3.0T, both of which were the most widespread magnetic resonance fields. And the variables were the ratios (NAA/Cr, NAA/Cho, NAA/ (Cho + Cr), Cho/Cr) of the 1H-MRS metabolites of the bilateral temporal poles at 1.5T and 3.0T. The Mann-Whitney U Test model was used in the 1.5T TLE group vs. 1.5T HCs and 3.0T TLE group vs. 3.0T HCs. The same healthy volunteer was scanned using 1.5T and 3.0T scanners, resulting in paired and correlated data. Therefore, the Pair T-Test was used to compare 3.0T HCs with 1.5T HCs. The difference is considered statistically significant if p <0.05. The power analysis was used to compare diagnostic capability differences between 1.5T and 3.0T scanners, and the probability of error, whose value depends on the significance criterion (), the sample size (N), and the population effect size (ES)[6] was assessed by the G*Power(version 3.1.9.7).Results
The results (Figure 4 (A-D) and Table 1(A-B)) of the Mann-Whitney U Test applied to 1.5T TLE group vs. 1.5T HCs and 3.0T TLE group vs. 3.0T HCs showed the same result that NAA/Cr, NAA/Cho, and NAA/(Cho + Cr) ratios of the TLE group were statistically different from the HCs in bilateral temporal poles, which demonstrated that three metabolic ratios (NAA/Cr, NAA/Cho, and NAA/(Cho+Cr)) might be used as potential biomarkers to identify the TLEs from the HCs. And the power analysis was used to evaluate metabolites’ ability to distinguish the TLE from HCs as well as both 1.5T and 3.0T scanners’ performances, i.e sensitivity and reproducibility (Table 1 (C)). Four metabolite ratios (NAA/Cr, NAA/Cho, NAA/(Cho+Cr), Cho/Cr) had similar distinction abilities with both 1.5T and 3.0T scanners, and validating the results of the Mann-Whitney U Test. As for 3.0T HCs vs 1.5T HCs, the results of the Paired T-Test showed (Figure 4(E-F) and Table 1 (D)) similar conclusion. Based on the result that NAA/Cr, NAA/Cho, and NAA/(Cho + Cr) were statistically significant at different field strengths, thus, 1.5T and 3.0T scanners might have comparable potential in distinguishing TLEs from HCs.Conclusion
To study the differences in the diagnostic utility of 1H-MRS for TLE at 1.5T and 3.0T, we designed four controlled experiments and adopted the Mann-Whitney U Test, the power analysis and the Paired T-Test. The potential biomarkers (NAA/Cr, NAA/Cho, NAA/ (Cho+Cr)) had the same utility using the 1.5T and 3.0T scanners for distinguishing the TLE from HCs in the bilateral temporal lobes. The power analysis showed similar statistic results on four metabolic ratios (NAA/Cr, NAA/Cho, NAA/(Cho+Cr), Cho/Cr). Additionally, metabolite ratios from the same healthy volunteers at 1.5T and 3.0T showed no significant difference. Given all the results discussed above, both 1.5T and 3.0T scanners may have comparable potential in distinguishing TLEs from HCs when 1H-MRS is used to identify patients with TLE.Acknowledgements
This study was financially supported by National Natural Science Foundation of China (62122064 and 61971361), Science and Technology Planning Project of Fujian Province (2020H6003), Xiamen Municipal Science and Technology Project (3502Z20193015), Fujian Provincial Health Young and Middle-aged Key Talents Training Project (2020GGB067), Fujian Health Education Joint Research Project (2019-WJ-31), Xiamen University Nanqiang Outstanding Talents, Fundamental Research Funds for the Central Universities (0621ZK1035), President Fund of Xiamen University. The correspondence should be sent to Yonggui Yang (Email: yangyonggui125@sina.cn), Gen Yan (Email: gyan@stu.edu.cn) and Xiaobo Qu (Email: quxiaobo@xmu.edu.cn)References
[1] M. J. Brodie and J. A. French, "Management of epilepsy in adolescents and adults," Lancet, vol. 356, no. 9226, pp. 323-329, 2000.
[2] R. D. Thijs, R. Surges, T. J. O'Brien, and J. W. Sander, "Epilepsy in adults," Lancet, vol. 393, no. 10172, pp. 689-701, 2019.
[3] A. Pitkänen et al., "Advances in the development of biomarkers for epilepsy," Lancet Neurol, vol. 15, no. 8, pp. 843-856, 2016.
[4] T. Hammen and R. Kuzniecky, "Magnetic resonance spectroscopy in epilepsy," Handbook of Clinical Neurology, vol. 107, pp. 399-408, 2012.
[5] D. Liu et al., "Brain metabolic differences between temporal lobe epileptic seizures and organic non-epileptic seizures in postictal phase: a retrospective study with magnetic resonance spectroscopy," Quantitative Imaging in Medicine and Surgery, vol. 11, no. 8, pp. 3781-3791, 2021.
[6] J. Cohen, "Statistical power analysis," Current Directions in Psychological Science, vol. 1, no. 3, pp. 98-101, 1992.
Figures