5215
Structural and functional changes in drug-naïve Rolandic epilepsy and their associated gene expression profiles
Yu Yin1 and Heng Liu1
1the Affiliated Hospital of Zunyi Medical University, Zunyi, China
1the Affiliated Hospital of Zunyi Medical University, Zunyi, China
Synopsis
Keywords: Epilepsy, fMRI (resting state)
Combining structural and functional neuroimaging analyses with brain transcriptional data, the present study investigated GMV and fALFF changes in children with RE as well as their underlying gene transcriptional profiles. VBM and fALFF analyses showed altered GMV and fALFF in brain regions associated with behavioral and cognitive regulation. Transcription-neuroimaging spatial correlation analyses further identified genes correlated with GMV and fALFF changes in RE, respectively. Moreover, functional enrichment analysis demonstrated that RE-related genes were enriched for the regulation of biological process. These findings may provide us with important knowledge for understanding the pathophysiological basis of this disease.Background
Rolandic epilepsy (RE) is a common pediatric epilepsy syndrome that has been widely reported to show abnormal brain structure and function. However, the genetic mechanisms underlying structural and functional changes remain largely unknown.Methods
Based on the structural and resting-state functional MRI data of 22 drug-naïve children with RE and 33 healthy controls, we conducted voxel-based morphology (VBM) and fractional amplitude of low-frequency fluctuation (fALFF) analyses to compared cortical morphology and spontaneous brain activity between the two groups. In combination with the Allen Human Brain Atlas, transcriptome-neuroimaging spatial correlation analyses were applied to exploring gene expression profiles associated with GMV and fALFF changes in RE.Results
VBM analysis demonstrated significantly increased GMV in the right brainstem and right middle cingulate gyrus in RE. Moreover, children with RE exhibited significantly increased fALFF in left temporal pole, while decreased fALFF in right thalamus and left precuneus. These brain structural and functional alterations were closely related to behavioral and cognitive deficits and the fALFF-linked gene expression profiles were enriched for cellular, metabolic as well as positive and negative regulations of biological process.Conclusion
Our findings suggest that brain morphological and functional abnormalities in children with RE involve complex polygenic genetic mechanisms.Acknowledgements
We thank the Allen Institute for Brain Science founders and staff who supplied the brain expression data. We also thank the subjects who contributed to this study. Address correspondence to Dr. Heng Liu, Department of Radiology, Affiliated Hospital of Zunyi Medical University, Medical Imaging Center of Guizhou Province, Zunyi 563003, China. Email: zmcliuh@163.com and Dr Jiaojian Wang, State Key Laboratory of Primate Biomedical Research, Institute of Primate Translational Medicine, Kunming University of Science and Technology, Kunming, China. Email: jiaojianwang@uestc.edu.cn.References
Arnatkeviciute A, Fulcher BD, Fornito A. 2019. A practical guide to linking brain-wide gene expression and neuroimaging data. Neuroimage. 189:353-367.Ashburner J, Friston KJ. 2000. Voxel-based morphometry--the methods. Neuroimage. 11:805-821.
Badawy RA, Freestone DR, Lai A, Cook MJ. 2012. Epilepsy: Ever-changing states of cortical excitability. Neuroscience. 222:89-99.
Berg AT, Berkovic SF, Brodie MJ, Buchhalter J, Cross JH, van Emde Boas W, Engel J, French J, Glauser TA, Mathern GW, Moshé SL, Nordli D, Plouin P, Scheffer IE. 2010. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology, 2005-2009. Epilepsia. 51:676-685.
Besseling RM, Jansen JF, Overvliet GM, van der Kruijs SJ, Vles JS, Ebus SC, Hofman PA, Louw A, Aldenkamp AP, Backes WH. 2013. Reduced functional integration of the sensorimotor and language network in rolandic epilepsy. Neuroimage Clin. 2:239-246.
Bobbili DR, Lal D, May P, Reinthaler EM, Jabbari K, Thiele H, Nothnagel M, Jurkowski W, Feucht M, Nürnberg P, Lerche H, Zimprich F, Krause R, Neubauer BA, Reinthaler EM, Zimprich F, Feucht M, Steinböck H, Neophytou B, Geldner J, Gruber-Sedlmayr U, Haberlandt E, Ronen GM, Altmüller J, Lal D, Nürnberg P, Sander T, Thiele H, Krause R, May P, Balling R, Lerche H, Neubauer BA. 2018. Exome-wide analysis of mutational burden in patients with typical and atypical Rolandic epilepsy. European journal of human genetics : EJHG. 26:258-264.
Bouma PA, Bovenkerk AC, Westendorp RG, Brouwer OF. 1997. The course of benign partial epilepsy of childhood with centrotemporal spikes: a meta-analysis. Neurology. 48:430-437.
Buckner RL, Andrews-Hanna JR, Schacter DL. 2008. The brain's default network: anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences. 1124:1-38.
Canario E, Chen D, Biswal B. 2021. A review of resting-state fMRI and its use to examine psychiatric disorders. Psychoradiology. 1:42-53.
Cavanna AE, Trimble MR. 2006. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 129:564-583.
Ciumas C, Montavont A, Ilski F, Laurent A, Saignavongs M, Lachaux JP, de Bellescize J, Panagiotakaki E, Ostrowsky-Coste K, Herbillon V, Ibarrola D, Hermier M, Arzimanoglou A, Ryvlin P. 2020. Neural correlates of verbal working memory in children with epilepsy with centro-temporal spikes. Neuroimage Clin. 28:102392.
Cloix JF, Hévor T. 2009. Epilepsy, regulation of brain energy metabolism and neurotransmission. Current medicinal chemistry. 16:841-853.
Dai XJ, Yang Y, Wang Y. 2022. Interictal epileptiform discharges changed epilepsy-related brain network architecture in BECTS. Brain Imaging Behav. 16:909-920.
Danielson NB, Guo JN, Blumenfeld H. 2011. The default mode network and altered consciousness in epilepsy. Behavioural neurology. 24:55-65.
Filippini M, Ardu E, Stefanelli S, Boni A, Gobbi G, Benso F. 2016. Neuropsychological profile in new-onset benign epilepsy with centrotemporal spikes (BECTS): Focusing on executive functions. Epilepsy Behav. 54:71-79.
Fritschy JM. 2008. Epilepsy, E/I Balance and GABA(A) Receptor Plasticity. Frontiers in molecular neuroscience. 1:5.
Georgiev D, Akram H, Jahanshahi M. 2021. Deep brain stimulation for psychiatric disorders: role of imaging in identifying/confirming DBS targets, predicting, and optimizing outcome and unravelling mechanisms of action. Psychoradiology. 1:118-151.
Girskis KM, Stergachis AB, DeGennaro EM, Doan RN, Qian X, Johnson MB, Wang PP, Sejourne GM, Nagy MA, Pollina EA, Sousa AMM, Shin T, Kenny CJ, Scotellaro JL, Debo BM, Gonzalez DM, Rento LM, Yeh RC, Song JHT, Beaudin M, Fan J, Kharchenko PV, Sestan N, Greenberg ME, Walsh CA. 2021. Rewiring of human neurodevelopmental gene regulatory programs by human accelerated regions. Neuron. 109:3239-3251.e3237.
Gong Y, Cai T. 1993. Manual of Wechsler Intelligence Scale for Children, Chinese revision (C-WISC).
Hawrylycz MJ, Lein ES, Guillozet-Bongaarts AL, Shen EH, Ng L, Miller JA, van de Lagemaat LN, Smith KA, Ebbert A, Riley ZL, Abajian C, Beckmann CF, Bernard A, Bertagnolli D, Boe AF, Cartagena PM, Chakravarty MM, Chapin M, Chong J, Dalley RA, David Daly B, Dang C, Datta S, Dee N, Dolbeare TA, Faber V, Feng D, Fowler DR, Goldy J, Gregor BW, Haradon Z, Haynor DR, Hohmann JG, Horvath S, Howard RE, Jeromin A, Jochim JM, Kinnunen M, Lau C, Lazarz ET, Lee C, Lemon TA, Li L, Li Y, Morris JA, Overly CC, Parker PD, Parry SE, Reding M, Royall JJ, Schulkin J, Sequeira PA, Slaughterbeck CR, Smith SC, Sodt AJ, Sunkin SM, Swanson BE, Vawter MP, Williams D, Wohnoutka P, Zielke HR, Geschwind DH, Hof PR, Smith SM, Koch C, Grant SGN, Jones AR. 2012. An anatomically comprehensive atlas of the adult human brain transcriptome. Nature. 489:391-399.
Herlin B, Navarro V, Dupont S. 2021. The temporal pole: From anatomy to function-A literature appraisal. Journal of chemical neuroanatomy. 113:101925.
Hu X, Tang J, Hua Y, Wang Y, Huang J. 2021. Evaluation of candidate genes in a Chinese cohort of atypical Rolandic epilepsy. Epileptic disorders : international epilepsy journal with videotape. 23:623-632.
Ismail FY, Fatemi A, Johnston MV. 2017. Cerebral plasticity: Windows of opportunity in the developing brain. Eur J Paediatr Neurol. 21:23-48.
Jurkevičienė G, Endzinienė M, Laukienė I, Šaferis V, Rastenytė D, Plioplys S, Vaičienė-Magistris N. 2012. Association of language dysfunction and age of onset of benign epilepsy with centrotemporal spikes in children. Eur J Paediatr Neurol. 16:653-661.
Kim EH, Yum MS, Shim WH, Yoon HK, Lee YJ, Ko TS. 2015. Structural abnormalities in benign childhood epilepsy with centrotemporal spikes (BCECTS). Seizure. 27:40-46.
Kovanich D, van der Heyden MA, Aye TT, van Veen TA, Heck AJ, Scholten A. 2010. Sphingosine kinase interacting protein is an A-kinase anchoring protein specific for type I cAMP-dependent protein kinase. Chembiochem : a European journal of chemical biology. 11:963-971.
Liu C, Han T, Xu Z, Liu J, Zhang M, Du J, Zhou Q, Duan Y, Li Y, Wang J, Cui D, Wang Y. 2022. Modulating Gamma Oscillations Promotes Brain Connectivity to Improve Cognitive Impairment. Cerebral cortex. 32:2644-2656.
Luo C, Zhang Y, Cao W, Huang Y, Yang F, Wang J, Tu S, Wang X, Yao D. 2015. Altered Structural and Functional Feature of Striato-Cortical Circuit in Benign Epilepsy with Centrotemporal Spikes. Int J Neural Syst. 25:1550027.
Ma MG, Liu XR, Wu Y, Wang J, Li BM, Shi YW, Su T, Li B, Liu DT, Yi YH, Liao WP. 2021. RYR2 Mutations Are Associated With Benign Epilepsy of Childhood With Centrotemporal Spikes With or Without Arrhythmia. Frontiers in neuroscience. 15:629610.
Matos M, Bara T, Clark S, Zeigelboim BS, Marques JM, Liberalesso PBN. 2018. Benign rolandic epilepsy of childhood and central auditory processing disorder: A noncasual neurophysiological association. Epilepsy Behav. 89:55-58.
McCormick DA, Contreras D. 2001. On the cellular and network bases of epileptic seizures. Annual review of physiology. 63:815-846.
Norden AD, Blumenfeld H. 2002. The role of subcortical structures in human epilepsy. Epilepsy Behav. 3:219-231.
Olson IR, Plotzker A, Ezzyat Y. 2007. The Enigmatic temporal pole: a review of findings on social and emotional processing. Brain. 130:1718-1731.
Pal DK, Ferrie C, Addis L, Akiyama T, Capovilla G, Caraballo R, de Saint-Martin A, Fejerman N, Guerrini R, Hamandi K, Helbig I, Ioannides AA, Kobayashi K, Lal D, Lesca G, Muhle H, Neubauer BA, Pisano T, Rudolf G, Seegmuller C, Shibata T, Smith A, Striano P, Strug LJ, Szepetowski P, Valeta T, Yoshinaga H, Koutroumanidis M. 2016. Idiopathic focal epilepsies: the "lost tribe". Epileptic disorders : international epilepsy journal with videotape. 18:252-288.
Pang Y, Zhao S, Li Z, Li N, Yu J, Zhang R, Lu F, Chen H, Wu F, Zheng W, Gao J, Yang Y, Wu H, Wang J. 2022. Enduring effect of abuse: Childhood maltreatment links to altered theory of mind network among adults. Human brain mapping. n/a.
Pardoe HR, Berg AT, Archer JS, Fulbright RK, Jackson GD. 2013. A neurodevelopmental basis for BECTS: evidence from structural MRI. Epilepsy Res. 105:133-139.
Rakhade SN, Jensen FE. 2009. Epileptogenesis in the immature brain: emerging mechanisms. Nat Rev Neurol. 5:380-391.
Romero-Garcia R, Warrier V, Bullmore ET, Baron-Cohen S, Bethlehem RAI. 2019. Synaptic and transcriptionally downregulated genes are associated with cortical thickness differences in autism. Molecular psychiatry. 24:1053-1064.
Roulet-Perez E, Mayor C. 2018. Childhood epilepsy with centro-temporal spikes (rolandic epilepsy) and written language. Dev Med Child Neurol. 60:219.
Rudolf G, de Bellescize J, de Saint Martin A, Arzimanoglou A, Valenti Hirsch MP, Labalme A, Boulay C, Simonet T, Boland A, Deleuze JF, Nitschké P, Ollivier E, Sanlaville D, Hirsch E, Chelly J, Lesca G. 2020. Exome sequencing in 57 patients with self-limited focal epilepsies of childhood with typical or atypical presentations suggests novel candidate genes. Eur J Paediatr Neurol. 27:104-110.
Siniatchkin M, Groening K, Moehring J, Moeller F, Boor R, Brodbeck V, Michel CM, Rodionov R, Lemieux L, Stephani U. 2010. Neuronal networks in children with continuous spikes and waves during slow sleep. Brain. 133:2798-2813.
Smith AB, Kavros PM, Clarke T, Dorta NJ, Tremont G, Pal DK. 2012. A neurocognitive endophenotype associated with rolandic epilepsy. Epilepsia. 53:705-711.
Specchio N, Wirrell EC, Scheffer IE, Nabbout R, Riney K, Samia P, Guerreiro M, Gwer S, Zuberi SM, Wilmshurst JM, Yozawitz E, Pressler R, Hirsch E, Wiebe S, Cross HJ, Perucca E, Moshé SL, Tinuper P, Auvin S. 2022. International League Against Epilepsy classification and definition of epilepsy syndromes with onset in childhood: Position paper by the ILAE Task Force on Nosology and Definitions. Epilepsia. 63:1398-1442.
Vears DF, Tsai MH, Sadleir LG, Grinton BE, Lillywhite LM, Carney PW, Harvey AS, Berkovic SF, Scheffer IE. 2012. Clinical genetic studies in benign childhood epilepsy with centrotemporal spikes. Epilepsia. 53:319-324.
Vogt BA. 2005. Pain and emotion interactions in subregions of the cingulate gyrus. Nat Rev Neurosci. 6:533-544.
Wang J, Becker B, Wang L, Li H, Zhao X, Jiang T. 2019. Corresponding anatomical and coactivation architecture of the human precuneus showing similar connectivity patterns with macaques. NeuroImage. 200:562-574.
Wang J, Wei Q, Bai T, Zhou X, Sun H, Becker B, Tian Y, Wang K, Kendrick K. 2017. Electroconvulsive therapy selectively enhanced feedforward connectivity from fusiform face area to amygdala in major depressive disorder. Social cognitive and affective neuroscience. 12:1983-1992.
Wang J, Yang Y, Zhao X, Zuo Z, Tan L-H. 2020. Evolutional and developmental anatomical architecture of the left inferior frontal gyrus. NeuroImage. 222:117268.
Wang L, Wei Q, Wang C, Xu J, Wang K, Tian Y, Wang J. 2020. Altered functional connectivity patterns of insular subregions in major depressive disorder after electroconvulsive therapy. Brain Imaging Behav. 14:753-761.
Xiao F, Li L, An D, Lei D, Tang Y, Yang T, Ren J, Chen S, Huang X, Gong Q, Zhou D. 2015. Altered attention networks in benign childhood epilepsy with centrotemporal spikes (BECTS): A resting-state fMRI study. Epilepsy Behav. 45:234-241.
Xie Z, Li J, Baker J, Eagleson KL, Coba MP, Levitt P. 2016. Receptor Tyrosine Kinase MET Interactome and Neurodevelopmental Disorder Partners at the Developing Synapse. Biol Psychiatry. 80:933-942.
Xu H, Zhu H, Luo L, Zhang R. 2021. Altered gray matter volume in MRI-negative focal to bilateral tonic-clonic seizures. Acta neurologica Belgica. 121:1525-1533.
Xu X, Li Q, Qian Y, Cai H, Zhang C, Zhao W, Zhu J, Yu Y. 2022. Genetic mechanisms underlying gray matter volume changes in patients with drug-naive first-episode schizophrenia. Cereb Cortex. bhac211.
Xue K, Liang S, Yang B, Zhu D, Xie Y, Qin W, Liu F, Zhang Y, Yu C. 2020. Local dynamic spontaneous brain activity changes in first-episode, treatment-naïve patients with major depressive disorder and their associated gene expression profiles. Psychological medicine.1-10.
Zang YF, He Y, Zhu CZ, Cao QJ, Sui MQ, Liang M, Tian LX, Jiang TZ, Wang YF. 2007. Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev. 29:83-91.
Zeng H, Ramos CG, Nair VA, Hu Y, Liao J, La C, Chen L, Gan Y, Wen F, Hermann B, Prabhakaran V. 2015. Regional homogeneity (ReHo) changes in new onset versus chronic benign epilepsy of childhood with centrotemporal spikes (BECTS): A resting state fMRI study. Epilepsy Res. 116:79-85.
Zhang J, Zhao T, Zhang J, Zhang Z, Li H, Cheng B, Pang Y, Wu H, Wang J. 2022. Prediction of childhood maltreatment and subtypes with personalized functional connectome of large-scale brain networks. Human brain mapping. 10.1002/hbm.25985..
Zhu Y, Yu Y, Shinkareva SV, Ji GJ, Wang J, Wang ZJ, Zang YF, Liao W, Tang YL. 2015. Intrinsic brain activity as a diagnostic biomarker in children with benign epilepsy with centrotemporal spikes. Hum Brain Mapp. 36:3878-3889.
Zou QH, Zhu CZ, Yang Y, Zuo XN, Long XY, Cao QJ, Wang YF, Zang YF. 2008. An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: fractional ALFF. Journal of neuroscience methods. 172:137-141.
Figures
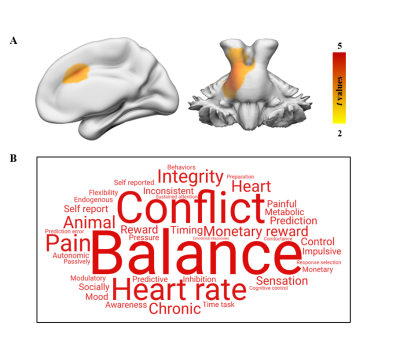
Figure
1. Functional decoding of brain regions
with differences in GMV. (A) VBM analysis shows GMV difference between the RE and HC groups. A cluster-level Monte Carlo simulation (1000 times)
corrected threshold of P < 0.05 was used to identify the significant
changes in GMV (cluster-forming threshold at voxel-level P < 0.001).
(B) Word clouds showing top cognitive terms associated with GMV differences
using the Neurosynth.
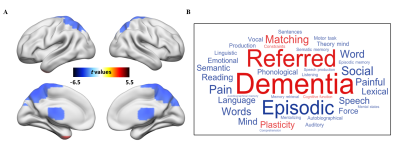
Figure 2.
Functional decoding of brain regions
with differences in fALFF. (A) Voxel-wise analysis shows fALFF difference
between the RE and HC groups. The significant level was determined using the
false discovery rate (FDR) method with P < 0.05. (B) Word clouds
showing top cognitive terms associated with fALFF differences using the Neurosynth.
Red and blue represent cognitive terms associated with regions showing
significantly higher and lower fALFF, respectively.
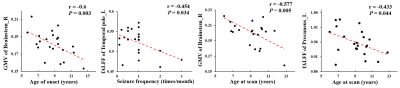
Figure 3. Correlation analyses between neuroimaging metrics and clinical characteristics. Correlation analyses between gray matter volume (GMV) values in right brainstem, fractional amplitude of low-frequency fluctuation (fALFF) in the left temporal pole and left precuneus and age of onset, age at scan, seizure frequency were performed. The significance was set at P < 0.05.
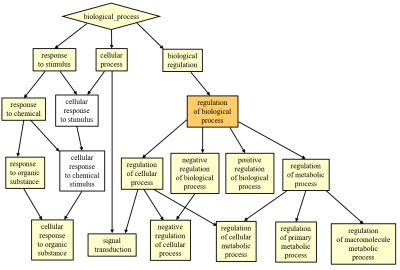
Figure 4.
Significant enrichment of ontology terms
for fALFF-associated genes in biological process. Colors indicate q
values for gene ontology terms.
DOI: https://doi.org/10.58530/2023/5215