5212
Focused Ultrasound Neuromodulation combined with rs-fMRI and EEG as a preclinical tool to investigate and intervene Drug-Induced Epilepsy
Yi-Jing Juan1, Xiao Zhen1, Po‑Chun Chu2, You-Yin Chen3, Hao-Li Liu2, and Jyh-Horng Chen2
1Graduate Institute of Biomedical Electronics and Bioinformatics, National Taiwan University, Taipei City, Taiwan, 2Department of Electrical Engineering, National Taiwan University, Taipei City, Taiwan, 3Department of Biomedical Engineering, National Yang Ming Chiao Tung University, Hsinchu City, Taiwan
1Graduate Institute of Biomedical Electronics and Bioinformatics, National Taiwan University, Taipei City, Taiwan, 2Department of Electrical Engineering, National Taiwan University, Taipei City, Taiwan, 3Department of Biomedical Engineering, National Yang Ming Chiao Tung University, Hsinchu City, Taiwan
Synopsis
Keywords: Epilepsy, fMRI (resting state), Focused Ultrasound, Neuromodulation, EEG
A drug-induced epileptic animal model to investigate the feasibility of FUS neuromodulation with rs-fMRI and EEGPurpose
Epilepsy is a neurological disorder characterized by abnormal neuro discharge [1]. A number of modalities has been developed to interfere epilepsy including deep brain stimulation, vagus nerve stimulation, and responsive neurostimulation [2]. As an alternative neuromodulation therapy, focused ultrasound (FUS) has found to have potential to modulate regional brain excitability [3], and recently burst-mode ultrasound stimulation has been shown to have epileptic suppressing effect [4]. In this study, we attempt to investigate the feasibility in utilizing resting-state functional MRI (rs-fMRI) and spontaneous neural activity to investigate the anti-epileptic effect induced by focused ultrasound pulsations in a drug-induced epileptic small-animal model.Methods
Sprague-Dawley rats were employed under the accordance of IACUC approved by National Taiwan University. Epileptic animal model was employed in this study by injecting pentylenetetrazol (PTZ) through intraperitoneal to trigger acute epileptic-like abnormal neuron discharges on the animal. MR-compatible polyimide-based microelectrodes [4] were implanted in the brain to record the neural activity in the thalamus. All neural data are recorded by the 4-channel Data Acquisition System MP36(BIOPAC Systems Inc., Goleta, California, USA). For rs-fMRI, image scans was performed in a 7 Tesla Bruker BioSpec MRI scanner (Bruker Corp., Billerica, MA, USA). All fMRI data are analyzed by SPM12 and Matlab scripts RESTplus to produce BOLD images. In order to minimize the image susceptibility in EPI caused by the neural implantation, animals were divided to neural recording group and fMRI group separately. In the neural recording animal group, their neural signals were recorded longitudinally to cover the PTZ injection and FUS pulsations, and were processed offline. In the rs-fMRI animal group, baseline fMRI was obtained, with the BOLD sequence was longitudinally to cover the entire FUS and epileptic onset duration (FUS pulsation is exposed to the model and observe the change of fMRI scan for 40 min). Ultrasound pulsations (1 MHz, 0.25 MI, duty cycle = 25%, exposure time = 10 min.) were introduced to observe the EEG and BOLD signals accordingly.Results
In neural recording experiments, there is no spikes or sharps waves found in the baseline data, but epileptic spikes were observed 10 min after PTZ injection (45±6.9 in 10-15 min), indicates the successfulness of the epilepsy establishment. Pulsed ultrasound was shown to successfully suppressed the epileptic onset in our previous identical setup [5]. In rs-fMRI group experiments, significant BOLD signal increases in the thalamus (from 0.052±0.024 to 0.224±0.068) and somatosensory cortex (from 0.259±0.056 to 0.408±0.062) 30 min after PTZ injection. FUS pulsations in animals also identified apparent FUS-modulated BOLD signals changes. Their corresponding BOLD signal changes in the thalamus increased from 0.068±0.013 to 0.734±0.062 post FUS pulsation 10 min. From the brain region correlation analysis to evaluate the functional connectivity change, it can be identified that both FUS pulsations and PTZ injections induced regions-to-region correlations change, whereas the correlations distribution were highly distinct.Conclusion
FUS pulsations provide great potentials to serve as an effective intervention tool to modulate the brain neuroactivity and has possibility to suppress epilepsy. We have demonstrated here that the approach via utilizing intracranial neural recording and rs-fMRI to assist the evaluation of anti-epileptic effect introduced by focused ultrasound pulsations is feasible. We also showed that longitudinal rs-fMRI monitoring and neural recording in the same epilepsy animal model have been successfully integrated to evaluate the drug-induced epileptic signal after PTZ injection. FUS induced neuromodulation induced region-to-region brain functional connectivity change, however further interpretation is necessary to understand the linkage behind the mechanism in using ultrasound to interfere epilepsy.Acknowledgements
No acknowledgement found.References
[1] P. Kwan et al. Expert Review of Neurotherapeutics 2006;6(3):397-406 [2] E. Jerome Neurology 2016;87(23):2483-2489 [3] R. F. Dallapiazza et al. JNS 2018;128(3):875-884 [4] S. G. Chen et al. Brain Stimulation 2020;13(1):35-46 [5] Y. Y. Chen et al. JNM 2009;182(1):6-16
Figures
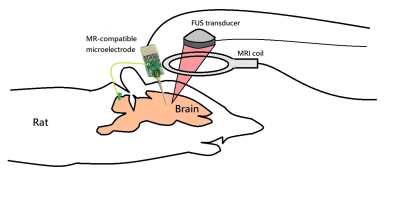
Figure 1. Experimental diagram of FUS
pulsations under the longitudinal monitoring of intracranial implanted EEG and
rs-fMRI
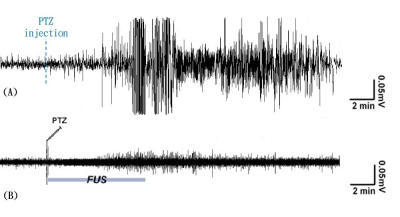
Figure 2. Comparison of brain wave patterns
between before and after FUS pulsations. (A) Animal with PTZ injection; (B)
animal with PTZ injection but was interfered by FUS pulsations [4]
Figure 3. longitudinal BOLD changes in (A)
normal animal, (B) animal with PTZ injection, and (C) animal with FUS
pulsations.
DOI: https://doi.org/10.58530/2023/5212