5211
Structural and functional changes in patients with temporal lobe epilepsy: a VBM and rs-fMRI study1Department of Magnetic Resonance, Lanzhou University Second Hospital, Lanzhou, China, 2Philips Healthcare, Xi'an, China
Synopsis
Keywords: Epilepsy, fMRI (resting state)
To investigate the structural and functional changes of the Temporal Lobe Epilepsy (TLE) patients, voxel-based morphometry (VBM) and resting-state functional MRI (rs-fMRI) methods were used. Our findings showed that thalamus gray matter volume decreased and performed negative correlation with self-rating anxiety scale (SAS) and self-rating depression scale (SDS). Furthermore, in rs-fMRI analysis, we found decreased functional connectivity between bilateral thalamus and right superior frontal gyrus. The results suggested that thalamus plays an important role in emotional change in TLE patients and structural and functional alterations may be related to the pathological mechanism of TLE.Introduction
Temporal lobe epilepsy (TLE) is a serious, common neurological disease1. Previous studies reported that widespread gray matter volume reduction in extratemporal structures in TLE patients2. And a few fMRI studies have already confirmed functional abnormalities in patients with TLE3. However, the inconsistent findings in structural and functional alterations of TLE make us hard to develop effective treatment. Therefore, by using voxel-based morphometry (VBM) and resting-state functional MRI (rs-fMRI) methods, we aim to explore the relationship between brain morphological abnormalities and brain functional connectivity changes in TLE patients and investigate the correlation between these alternations and clinical scales to find imaging markers of TLE. This work intends to provide imaging evidence for further exploration of abnormal electrical activity propagation in brain to understand the pathological mechanism of TLE.Methods
This study included 40 patients with clinically diagnosed TLE and 31 age-, sex- and educational matched healthy controls (HC). For all enrolled participants, self-rating anxiety scale (SAS) and self-rating depression scale (SDS) were tested to evaluate their anxiety and depression. Table 1 showed the demographic and clinical characteristics of TLE and HC (Table.1). All images were obtained on a 3T scanner (Ingenia CX, Philips Healthcare, the Netherlands) with a 32-channel phase array head coil. High resolution sagittal 3D T1 imaging was used for structural data collection. The corresponding parameters were as follows: TR=3900ms, TE=3.5ms, flip angle=12°, 360slices, 1x1x1 mm3 spatial resolution. Conventional single shot EPI BOLD imaging was applied for functional data acquisition. The corresponding parameters were: TR=1000ms, TE=20ms, 48slices, 3x3x3 mm3 spatial resolution, 128x128 in-plane matrix, scan time=4imn 48s. VBM was used to explore the changes of gray matter volume (GMV) in TLE. Regions showing abnormal GMV in patients were adopted as region of interests (ROIs) for the functional connectivity (FC) analysis. The structural and functional images were preprocessed by using SPM12 (https://www.êl.ion.ucl.ac.uk/spm/) and DPABI (http://www.rfmri.org/dpabi) software. Then two-sample t test was performed to find the group difference. Multiple comparison (false discovery rate, FDR) correction was performed to avoid false positives. Finally, Spearman correlation analysis was used to explore the GMV and their correlation with SAS and SDS, P < 0.05 was reported as statistically significant.Results
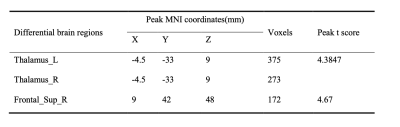
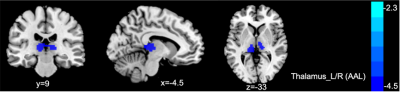
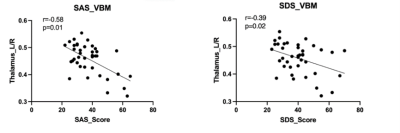
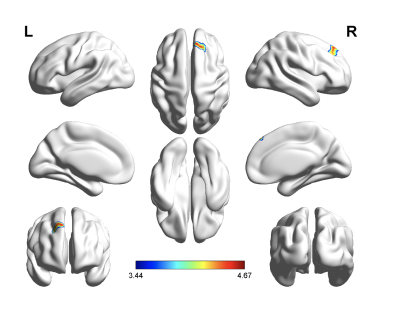
Demographic and Clinical Characteristics were shown in table1. The two groups did not differ in terms of age, gender, or educational distribution. Compared with the HC, patients with TLE showed a significantly higher SDS score (p < 0.001) and SAS scores (p < 0.001). Table 2 showed the statistical results of VBM and FC between TLE and HC group. Compared with HC, patients with TLE showed significantly decreased GMV in the bilateral thalamus (Fig 1, Table 2). A negative correlation was found between GMV in the bilateral thalamus and the SAS and SDS in patients with TLE (Fig 2, Table 2). Furthermore, compared with HC, patients with TLE showed significantly weaker FC between the thalamus and right superior frontal gyrus (Fig 3, Table 2). No significant difference results were found in HC group.Discussion
In this study, we found that the volume reduced in thalamus in patient with TLE compared with HC. Besides, with the decreasing volume, anxiety and depression degree were increased in TLE. As a part of the epileptic network activated during temporal lobe seizures, thalamus’ atrophy represents the most common extratemporal abnormality. As a relay station for epileptic activity, the thalamus contributes to motor planning, language, emotion and memory by promoting cortical synchronization and facilitating cortico-cortical interplay4. Therefore, in our study, anxiety and depression in patients with TLE may be related to changes in their thalamus. The frontal cortex showed a pathological covariation with the thalamus in TLE, which is caused by seizure propagation across these two structures5. The decreased FC between thalamus and prefrontal cortex may relate to derangements of cortico-subcortical connectivity. Our study supported the hypothesis that the prefrontal cortex-thalamus pathway may be an important structure for exploring transmission of electrical activity in TLE.Conclusion
Our results indicated the central role of the thalamus in patients with TLE. The thalamus may be related to anxiety and depression in TLE patients. Furthermore, decreased functional connectivity between the thalamus and prefrontal cortex may be involved in the spread of electrical activity. Thalamus abnormality may be the important imaging biomarker for clinical evaluation and treatment of TLE.Acknowledgements
This work was supported by the Natural Science Foundation of China (Grant No. 82160326).References
1. MUHLHOFER W, TAN Y-L, MUELLER S G, et al. MRI-negative temporal lobe epilepsy—What do we know? [J]. Epilepsia, 2017, 58(5): 727-42.
2. ALVIM M K M, COAN A C, CAMPOS B M, et al. Progression of gray matter atrophy in seizure-free patients with temporal lobe epilepsy [J]. Epilepsia, 2016, 57(4): 621-9.
3. HE X, DOUCET G, SPERLING M, et al. Reduced thalamocortical functional connectivity in temporal lobe epilepsy [J]. Epilepsia, 2015, 56(10): 1571-9.
4. CACIAGLI L, ALLEN L, HE X, et al. Thalamus and focal to bilateral seizures: A multiscale cognitive imaging study [J]. Neurology, 2020, 95(17): e2427-e41.
5. ZHANG C, ZHANG H, XU K, et al. Impaired prefrontal cortex-thalamus pathway in intractable temporal lobe epilepsy with aberrant executive control function: MRI evidence [J]. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology, 2019, 130(4): 484-90.
Figures